425: Filing under Securities Act Rule 425 of certain prospectuses and communications in connection with business combination transactions
Published on October 10, 2014

Vince
Forlenza CEO and Chairman
BD
FILED
BY
BECTON,
DICKINSON
AND
COMPANY
PURSUANT
TO
RULE
425
UNDER
THE
SECURITIES
ACT
OF
1933
AND
DEEMED
FILED
PURSUANT
TO
RULE
14A-12
OF
THE
SECURITIES
EXCHANGE
ACT
OF
1934
SUBJECT
COMPANY:
CAREFUSION
CORP
COMMISSION
FILE
NO.
001-34273 |

2
BD is a company with strong values and a track record of success
Announcement accelerates CareFusions plans to build greater scale
New growth opportunities for products in emerging markets and
expands global reach by leveraging BDs international infrastructure
Offers a complete suite of medication management and patient safety
solutions
The hard work of CareFusions 17,000 employees got us here today
Key Highlights |
3
BD is a medical technology company that serves healthcare institutions, life
science researchers, clinical laboratories, industry and the general public.
Manufactures and sells a broad range of medical supplies, devices, laboratory
equipment and diagnostic products
Innovative solutions are focused on improving drug delivery, enhancing the
diagnosis of infectious diseases and cancers, supporting the management of
diabetes and advancing cellular research
30,000 employees in 50 countries who strive to fulfill the companys purpose
of Helping all people live healthy lives
HQ in Franklin Lakes, New Jersey
Who is BD?
A Leading Global Medical Technology Company |
4
1897
Company founding
1906
First U.S. syringe and needle factory
1924
First insulin injection device
1942
First penicillin injection device
1949
First evacuated blood collection tube
1952
First sterile disposable device
1954
First disposable syringes for Salk Polio campaign
1962
First mass produced syringes and needles
1968
First automated blood culture system
1972
First fluorescence activated cell sorter
1988
First safety-engineered syringe
1991
First auto-disable syringe
1995
BD Insyte
Autoguard
IV Catheter
2002
BD FACSAria
Cell Sorter
2006
GeneOhm (HAIs) and TriPath (cancer Dx)
2012
KIESTRA Lab Automation
BD Legacy of Health Impact |

5
Advancing the quality, accessibility, safety and affordability of
healthcare around the world
Long-standing relationships with non-governmental organizations
Social investment includes corporate grants, product donations,
volunteer service initiatives and disaster relief programs
Donate time, technical expertise and resources
Associate engagement programs
International volunteer opportunities
Helping all people live healthy lives |

6
118 years of successful legacy in the healthcare industry
More than just a world-class quality medical technology
company
Committed to addressing customer needs and foremost
health priorities around the world
Conclusions |

Tom
Polen Segment President
BD Medical |
8
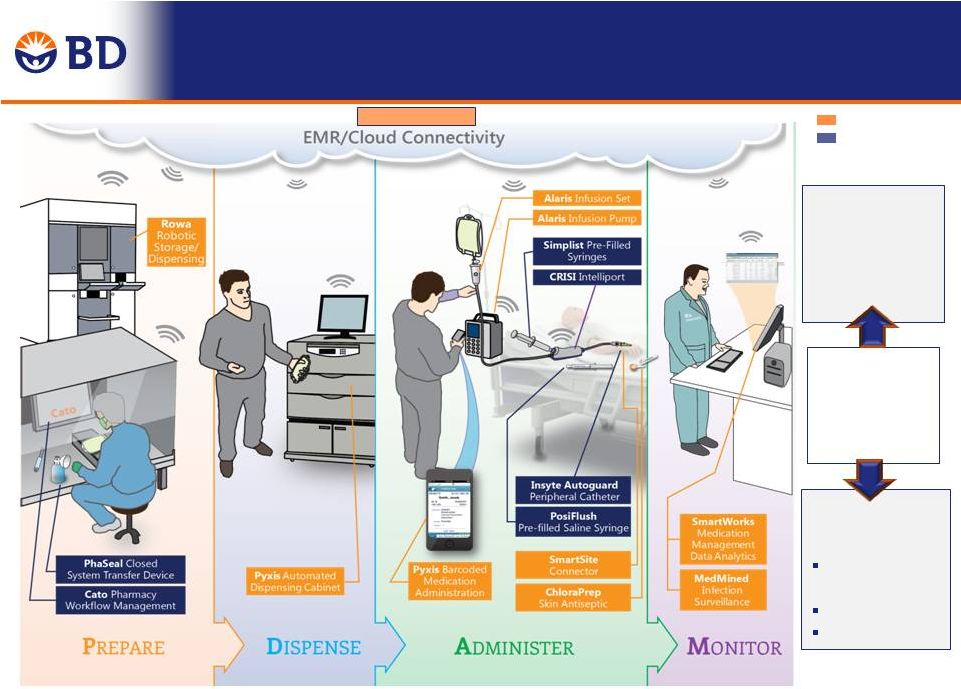
CareFusion Portfolio
BD Portfolio
In Medication Management, Our Combined Portfolios
Provide an End-to-End Solution from Pharmacy to Patient
End-to-End
medication
management
Solution
Reduction in
Costs
Labor
Efficiency
Drug Waste
Inventory
Smartworks
Improvement
in Quality
Med Errors
Infections
TM |
9
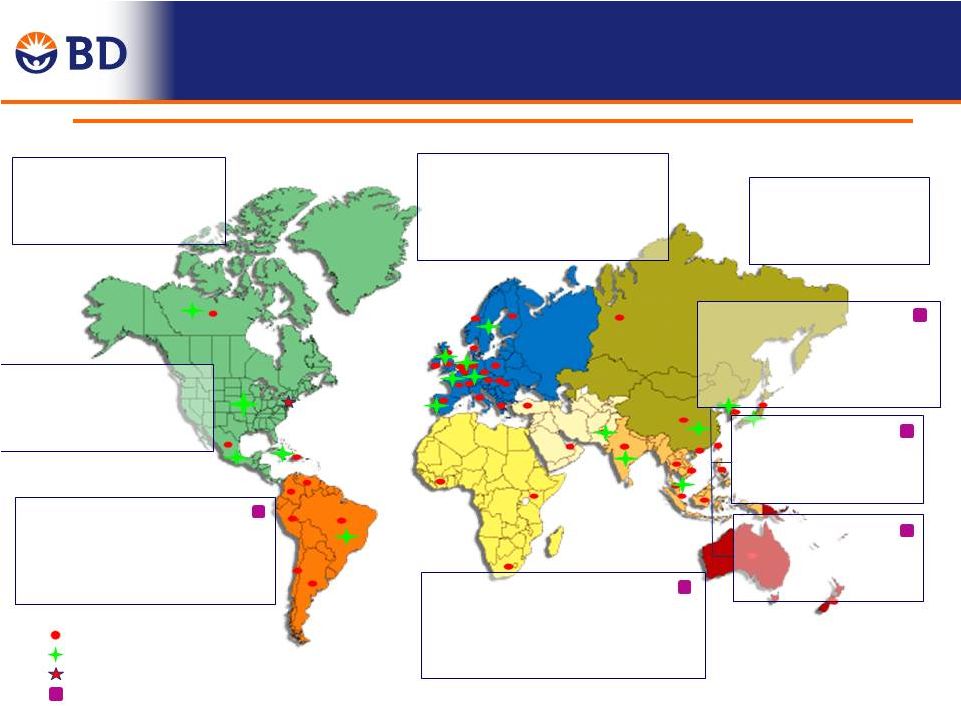
Leading Global Infrastructure enables
accelerated international expansion
Updated September 2013
BD in Western Europe
First
location
-
France
-
1952
Legal Entities in 21 countries
Ten plants
6,000 BD associates
BD in Latin America
First
location
-
Mexico
1952
Legal entities in eight countries
Five plants
5,200 BD associates
BD in Asia Pacific
BD In Japan
Established -
1971
One plant
600 BD associates
BD in EMA
First Location
Dubai, UAE
1994
Legal entities in ten countries
No manufacturing
250 BD associates
Legal Entities
Plant locations
Corporate Office
Emerging Markets
BD In India
Established -
1995
One plant
550 BD associates
BD In China
Established -
1994
Two plants
2,200 BD associates
BD in Canada
Established
1951
One plant
550 BD associates
BD in United States
Established
1897
29 plants
12,000 BD associates
First
location
Australia,1971
Legal entities in 16 countries
Five plants
4,600 BD associates |

Bill
Kozy COO
BD |
11
Phases of integration
Timing
Phase I
Phase II
Listen, engage (and focus
on business momentum)
Jointly plan and
countdown to close
Transition to NewCo
2-4+ months
Key
Activities
First ~30 days
Close to 2-3 years
Create the integration
architecture and launch
the integration teams
(IMO, value capture and
functional)
Develop talent selection,
retention and business
protection approach
Initiate baseline creation
and target setting
Launch culture
assessment and frequent
communications
Develop Day 1/100 plans
Develop detailed synergy
ideas and value creation
plans
Countdown to close: lock
targets, finalize plans and
prepare for Day 1
Leverage culture insights
in org design and Day 1
plans
Execute Day 1/100 plans,
onboard employees, track
progress
Ensure culture integration
and execute interventions
Phase III |
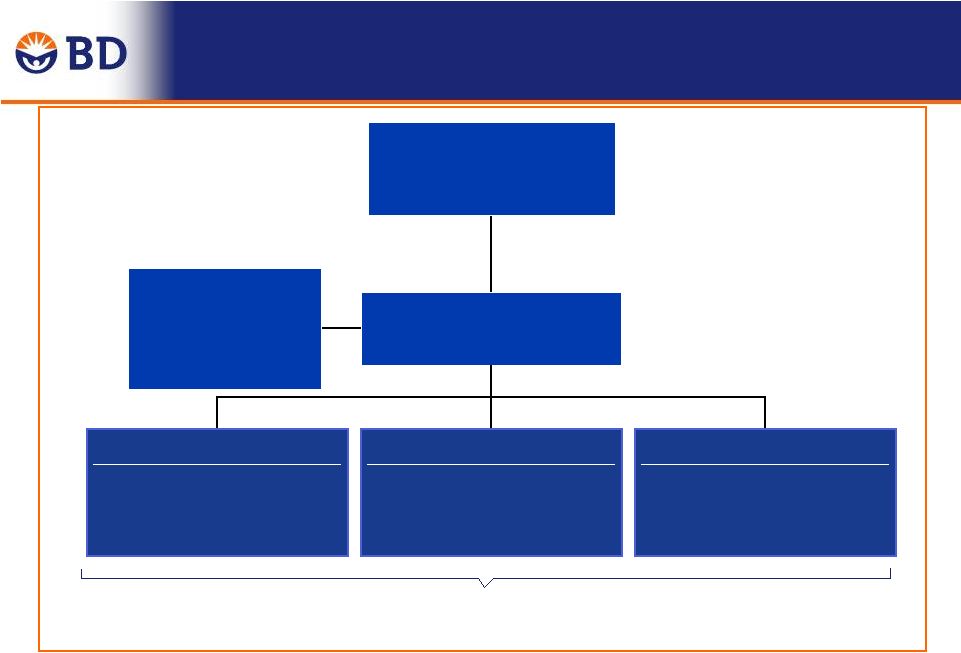
12
Integration project structure
Integration Management Office
Tom Jaeger
Chair
CareFusion
Vice Chair
Value Capture
Advisory Council
Bill Kozy
Chair
Chairmans Integration
Council
Vince Forlenza
Chair
Bill Kozy
Vice Chair
Function teams
Create NewCo infrastructure
and capture cost synergies
BU/Geographic teams
Capture business value
Issue resolution teams
Resolve targeted issues
Team members drawn from both companies |

|
Forward-Looking Statements
This communication contains certain estimates and other forward-looking statements (as defined under Federal securities laws). Forward looking statements generally are accompanied by words such as will, expect, outlook or other similar words, phrases or expressions. These forward-looking statements include statements regarding the estimated or anticipated future results of BD, and of the combined company following BDs proposed acquisition of CareFusion, the anticipated benefits of the proposed combination, including estimated synergies, the expected timing of completion of the transaction and other statements that are not historical facts. These statements are based on the current expectations of BD and CareFusion management and are not predictions of actual performance. These statements are subject to a number of risks and uncertainties regarding BD and CareFusions respective businesses and the proposed acquisition, and actual results may differ materially. These risks and uncertainties include, but are not limited to, the ability of the parties to successfully close the proposed acquisition, including the risk that the required regulatory approvals are not obtained, are delayed or are subject to unanticipated conditions that could adversely affect the combined company or the expected benefits of the transaction; risks relating to the integration of CareFusions operations, products and employees into BD and the possibility that the anticipated synergies and other benefits of the proposed acquisition will not be realized or will not be realized within the expected timeframe; the outcome of any legal proceedings related to the proposed merger; access to available financing for the refinancing of BDs or CareFusions debt on a timely basis and reasonable terms; the ability to market and sell CareFusions products in new markets, including the ability to obtain necessary regulatory product registrations and clearances; the loss of key senior management or other associates; the anticipated demand for BDs and CareFusions products, including the risk of future reductions in government healthcare funding, changes in reimbursement rates or changes in healthcare practices that could result in lower utilization rates or pricing pressures; the impact of competition in the medical device industry; the risks of fluctuations in interest or foreign currency exchange rates; product liability claims; difficulties inherent in product development, including the timing or outcome of product development efforts, the ability to obtain regulatory approvals and clearances and the timing and market success of product launches; risks relating
to fluctuations in the cost and availability of raw materials and other sourced products and the ability to maintain favorable supplier arrangements and relationships; successful compliance with governmental regulations applicable to BD, CareFusion and the combined company; changes in regional, national or foreign economic conditions; uncertainties of litigation, as well as other factors discussed in BDs and CareFusions respective filings with the Securities Exchange Commission. BD and CareFusion do not intend to update any forward-looking statements to reflect events or circumstances after the date hereof, except as required by applicable laws or regulations.
IMPORTANT INFORMATION FOR INVESTORS
In connection with the proposed transaction, BD will file with the SEC a registration statement on Form S4 that will constitute a prospectus of BD and include a proxy statement of CareFusion. BD and CareFusion also plan to file other relevant documents with the SEC regarding the proposed transaction. INVESTORS ARE URGED TO READ THE PROXY STATEMENT/PROSPECTUS AND OTHER RELEVANT DOCUMENTS FILED WITH THE SEC IF AND WHEN THEY BECOME AVAILABLE, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION. You may obtain a free copy of the proxy statement/prospectus (if and when it becomes available) and other relevant documents filed by BD and CareFusion with the SEC at the SECs website at www.sec.gov. In addition, you will be able to obtain free copies of these documents by phone, email or written request by contacting the investor relations department of BD or CareFusion at the following: Monique N. Dolecki, Investor Relations 201-847-5378 Monique_Dolecki@bd.com or Jim Mazzola, Investor Relations 858-617-1203 Jim.Mazzola@CareFusion.com
PARTICIPANTS IN THE SOLICITATION
BD and CareFusion and their respective directors and executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies in respect of the proposed transaction. Information about BDs directors and executive officers is available in BDs proxy statement dated December 19, 2013, for its 2014 Annual Meeting of Shareholders and subsequent SEC filings. Information about CareFusions directors and executive officers is available in CareFusions proxy statement dated September 25, 2014, for its 2014 Annual Meeting of Stockholders. Other information regarding the participants in the proxy solicitation and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the proxy statement/prospectus and other relevant materials to be filed with the SEC regarding the merger when they become available. Investors should read the proxy statement/prospectus carefully when it becomes available before making any voting or investment decisions. You may obtain free copies of these documents from BD or CareFusion as indicated above. This communication shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the U.S. Securities Act of 1933, as amended.
2