Published on November 23, 2007
Exhibit 13
| Financials |
Becton, Dickinson and Company
|
| Financial Table of Contents | Page | |
| Ten-Year Summary of Selected Financial Data | 18 | |
| Financial Review | 20 | |
| Reports of Management | 33 | |
| Reports of Independent Registered Public Accounting Firm | 34 | |
| Consolidated Statements of Income | 36 | |
| Consolidated Statements of Comprehensive Income | 37 | |
| Consolidated Balance Sheets | 38 | |
| Consolidated Statements of Cash Flows | 39 | |
| Notes to Consolidated Financial Statements | 40 | |
| Quarterly Data (unaudited) | 62 |
17
| Summary |
Becton, Dickinson and Company
|
Ten-Year Summary of Selected Financial Data
Years Ended September 30
Dollars in millions, except per share amounts
| 2007 | 2006 | 2005 | 2004 |
|
||||||||||||
| Operations |
|
|||||||||||||||
| Revenues | $ | 6,359.7 | $ | 5,738.0 | $ | 5,340.8 | $ | 4,893.9 |
|
|||||||
| Research and Development Expense | 360.1 | 301.9 | 267.7 | 230.8 |
|
|||||||||||
| Operating Income | 1,203.2 | 1,141.4 | 1,063.8 | 878.2 |
|
|||||||||||
| Interest Expense, Net | .2 | 6.8 | 19.3 | 29.6 |
|
|||||||||||
| Income From Continuing Operations |
|
|||||||||||||||
| Before Income Taxes | 1,203.9 | 1,125.9 | 1,037.5 | 843.8 |
|
|||||||||||
| Income Tax Provision | 347.8 | 310.8 | 325.0 | 204.9 |
|
|||||||||||
| Net Income | 890.0 | 752.3 | 722.3 | 467.4 |
|
|||||||||||
| Basic Earnings per Share | 3.63 | 3.04 | 2.87 | 1.85 |
|
|||||||||||
| Diluted Earnings per Share | 3.49 | 2.93 | 2.77 | 1.77 |
|
|||||||||||
| Dividends per Common Share | .98 | .86 | .72 | .60 |
|
|||||||||||
| Financial Position |
|
|||||||||||||||
| Current Assets | $ | 3,130.6 | $ | 3,185.3 | $ | 2,975.3 | $ | 2,641.3 |
|
|||||||
| Current Liabilities | 1,478.8 | 1,576.3 | 1,299.4 | 1,050.1 |
|
|||||||||||
| Property, Plant and Equipment, Net | 2,497.3 | 2,133.5 | 1,933.7 | 1,881.0 |
|
|||||||||||
| Total Assets | 7,329.4 | 6,824.5 | 6,132.8 | 5,752.6 |
|
|||||||||||
| Long-Term Debt | 955.7 | 957.0 | 1,060.8 | 1,171.5 |
|
|||||||||||
| Shareholders' Equity | 4,362.0 | 3,836.2 | 3,284.0 | 3,067.9 |
|
|||||||||||
| Book Value per Common Share | 17.89 | 15.63 | 13.26 | 12.30 |
|
|||||||||||
| Financial Relationships |
|
|||||||||||||||
| Gross Profit Margin | 51.7 | % | 51.3 | % | 50.9 | % | 50.5 |
% |
||||||||
| Return on Revenues(E) | 13.5 | % | 14.2 | % | 13.3 | % | 13.1 |
% |
||||||||
| Return on Total Assets(B)(E) | 17.7 | % | 18.4 | % | 18.4 | % | 15.7 |
% |
||||||||
| Return on Equity(E) | 20.9 | % | 22.9 | % | 22.4 | % | 21.4 |
% |
||||||||
| Debt to Capitalization(D)(E) | 20.9 | % | 25.8 | % | 27.1 | % | 28.1 |
% |
||||||||
| Additional Data |
|
|||||||||||||||
| Number of Employees | 28,000 | 27,000 | 25,600 | 25,000 |
|
|||||||||||
| Number of Shareholders | 8,896 | 9,147 | 9,442 | 9,654 |
|
|||||||||||
| Average Common and Common |
|
|||||||||||||||
| Equivalent Shares Outstanding |
|
|||||||||||||||
| Assuming Dilution (millions) | 254.8 | 256.6 | 260.7 | 263.3 |
|
|||||||||||
| Depreciation and Amortization | $ | 441.3 | $ | 402.3 | $ | 382.7 | $ | 351.1 |
|
|||||||
| Capital Expenditures | 556.4 | 457.1 | 315.8 | 260.5 |
|
|||||||||||
| (A) |
Includes cumulative effect of accounting change of $36.8 million ($.14 per basic and diluted share). |
| (B) |
Earnings before interest expense, taxes and cumulative effect of accounting changes as a percent of average total assets. |
| (C) |
Excludes the cumulative effect of accounting changes. |
| (D) |
Total debt as a percent of the sum of total debt, shareholders' equity and net non-current deferred income tax liabilities. |
| (E) |
Excludes discontinued operations in 1999 to 2007. |
18
Becton, Dickinson and Company
| 2003 | 2002 | 2001 | 2000 | 1999 | 1998 | |||||||||||||||||
| $ | 4,449.1 | $ | 3,960.4 | $ | 3,667.6 | $ | 3,544.7 | $ | 3,412.6 | $ | 3,116.9 | |||||||||||
| 218.5 | 201.1 | 193.8 | 207.8 | 203.9 | 187.9 | |||||||||||||||||
| 800.8 | 689.1 | 645.9 | 507.4 | 477.3 | 405.4 | |||||||||||||||||
| 36.5 | 33.2 | 55.3 | 74.2 | 72.0 | 56.3 | |||||||||||||||||
| 761.6 | 642.1 | 548.6 | (A) | 512.7 | 404.8 | 340.9 | ||||||||||||||||
| 182.1 | 153.7 | 139.3 | 122.0 | 96.9 | 104.3 | |||||||||||||||||
| 547.1 | 480.0 | 401.7 | (A) | 392.9 | 275.7 | 236.6 | ||||||||||||||||
| 2.14 | 1.85 | 1.55 | (A) | 1.54 | 1.09 | .95 | ||||||||||||||||
| 2.07 | 1.79 | 1.49 | (A) | 1.49 | 1.04 | .90 | ||||||||||||||||
| .40 | .39 | .38 | .37 | .34 | .29 | |||||||||||||||||
| $ | 2,503.5 | $ | 2,091.4 | $ | 1,930.1 | $ | 1,847.6 | $ | 1,843.0 | $ | 1,542.8 | |||||||||||
| 1,059.4 | 1,271.5 | 1,285.4 | 1,382.4 | 1,358.6 | 1,091.9 | |||||||||||||||||
| 1,831.8 | 1,750.4 | 1,701.3 | 1,565.5 | 1,423.9 | 1,302.7 | |||||||||||||||||
| 5,572.3 | 5,029.0 | 4,790.8 | 4,505.1 | 4,437.0 | 3,846.0 | |||||||||||||||||
| 1,184.0 | 803.0 | 782.8 | 778.5 | 954.0 | 765.2 | |||||||||||||||||
| 2,897.0 | 2,480.9 | 2,321.7 | 1,956.0 | 1,768.7 | 1,613.8 | |||||||||||||||||
| 11.54 | 9.71 | 8.96 | 7.72 | 7.05 | 6.51 | |||||||||||||||||
| 48.9 | % | 48.3 | % | 48.7 | % | 48.6 | % | 49.9 | % | 50.6 | % | |||||||||||
| 13.0 | % | 12.3 | % | 12.2 | %(C) | 11.0 | % | 9.0 | % | 7.6 | % | |||||||||||
| 15.2 | % | 13.9 | % | 13.9 | % | 13.4 | % | 11.6 | % | 11.7 | % | |||||||||||
| 21.6 | % | 20.3 | % | 20.7 | %(C) | 21.0 | % | 18.2 | % | 15.8 | % | |||||||||||
| 30.5 | % | 32.7 | % | 34.0 | % | 41.7 | % | 47.6 | % | 41.4 | % | |||||||||||
| 24,800 | 25,200 | 24,800 | 25,000 | 24,000 | 21,700 | |||||||||||||||||
| 9,868 | 10,050 | 10,329 | 10,822 | 11,433 | 9,784 | |||||||||||||||||
| 263.6 | 268.2 | 268.8 | 263.2 | 264.6 | 262.1 | |||||||||||||||||
| $ | 332.8 | $ | 294.7 | $ | 292.0 | $ | 273.7 | $ | 257.8 | $ | 228.7 | |||||||||||
| 253.0 | 253.5 | 364.1 | 371.0 | 311.4 | 181.4 | |||||||||||||||||
19
| Financial Review |
Becton, Dickinson and Company
|
Company Overview
Becton, Dickinson and Company (BD) is a medical technology company engaged principally in the manufacture and sale of a broad range of medical supplies, devices, laboratory equipment and diagnostic products used
by healthcare institutions, life science researchers, clinical laboratories, industry and the general public. Our business consists of three worldwide business segmentsBD Medical (Medical), BD Diagnostics (Diagnostics)
and BD Biosciences (Biosciences). Our products are marketed in the United States and internationally through independent distribution channels, directly to end-users and by independent sales representatives. References to years
throughout this discussion relate to our fiscal years, which end on September 30.
BD management operates the business consistent with the following core strategies:
-
To increase revenue growth by focusing on products that deliver greater benefits to patients, healthcare workers and researchers;
-
To improve operating effectiveness and balance sheet productivity; and,
-
To strengthen organizational and associate capabilities in the ever-changing healthcare environment.
In assessing the outcomes of these strategies and BDs financial condition and operating performance, management generally reviews quarterly forecast data, monthly actual results, segment sales and other similar information. We also consider trends related to certain key financial data, including gross profit margin, selling and administrative expense, investment in research and development, and cash flows.
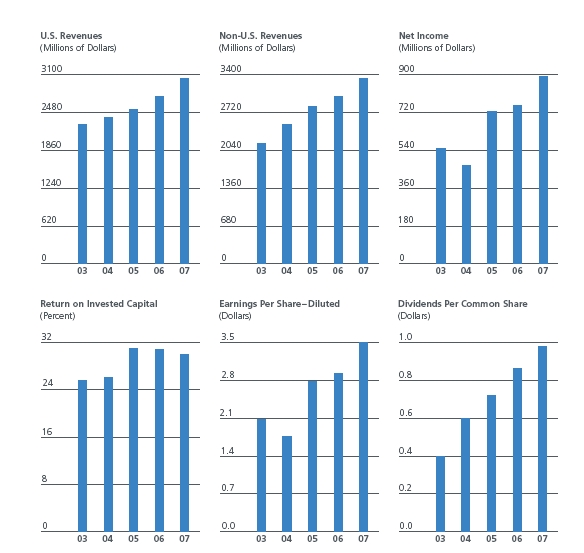
The results of our strategies are reflected in our fiscal 2007 financial and operational performance. Worldwide revenues in 2007 of $6.4 billion increased 11% from the prior year and reflected volume increases of approximately 8%, an estimated increase due to favorable foreign currency translation of 3%, and price increases of less than 1%. U.S. revenues increased 11% to $3.0 billion. International revenues increased 11% to $3.3 billion with an estimated 5 percentage points of such growth coming from the favorable impact from foreign currency. For a discussion of the financial impact of exchange rate fluctuations and the ways and extent to which we attempt to mitigate such impact, see Financial Instrument Market Risk below.
Consistent with our strategy to provide products that deliver greater benefits to healthcare workers, and recognizing the issues surrounding sharps-related injuries, BD has developed a wide array of safety-engineered devices that are designed to reduce the incidence of needlestick injuries and exposure to bloodborne pathogens. These products are offered through our Medical and Diagnostics segments. Sales in the United States of safety-engineered devices grew 7% to $982 million in 2007, from $917 million in 2006. International sales of safety-engineered devices grew 26% to $409 million in 2007 from $324 million in 2006. In 2008, we expect sales of safety-engineered devices to increase about 8% in the United States and 20% internationally.
Income from Continuing Operations was $856 million, or $3.36 per diluted share, in 2007 as compared with $815 million, or $3.18 per diluted share, in 2006. Comparisons of Income from Continuing Operations between 2007 and 2006 are affected by the following significant items that are reflected in our financial results:
2007
-
In December 2006, we acquired TriPath Imaging, Inc. (TriPath). TriPath develops, manufactures, markets and sells innovative solutions to improve the clinical management of cancer, including detection, diagnosis, staging and treatment. In connection with the acquisition, we incurred a pre-tax non-cash charge of $115 million, or $.45 per diluted share, for acquired in-process research and development.
-
In December 2006, we sold the blood glucose monitoring (BGM) product line. Following the sale, prior period Consolidated Statements of Income and Cash Flows were restated to separately present the results of the BGM product line as discontinued operations.
2006
-
In February 2006, we acquired GeneOhm Sciences, Inc. (GeneOhm). In connection with the acquisition, we incurred a pre-tax non-cash charge of $53 million, or $.21 per diluted share, for acquired in-process research and development.
Our ability to sustain our long-term growth will depend on a number of factors, including our ability to expand our core business (including geographical expansion), develop innovative new products with higher gross profit margins across our business segments, and continue to improve operating efficiency and organizational effectiveness. Numerous factors can affect our ability to achieve these goals including, without limitation, economic conditions in the United States and elsewhere, increased competition and healthcare cost containment initiatives.
20
Financial Review Becton, Dickinson and Company
We believe several important factors relating to our business tend to reduce the impact on BD of any potential economic or political events in countries in which we do business, including the effects of possible healthcare system reforms. For example, since many of our products are used in essential medical care, demand for such products tends not to be significantly affected by economic fluctuations. Other factors include the international nature of our business and our ability to meet the needs of the worldwide healthcare industry with cost-effective and innovative products.
In 2007, general inflation did not have a material impact on our overall operations. However, it is possible that general inflation rates will rise in 2008 and beyond, and could have a greater impact on worldwide economies and, consequently, on BD. BD purchases supplies of resins, which are oil-based components used in the manufacture of certain products. During 2007, we incurred slightly higher resin purchase costs than the prior year, primarily due to increases in world oil prices during the late summer 2006. Such increases did not have a significant impact on our 2007 operating results. Any significant increases in resin purchase costs could impact future operating results.
Our anticipated revenue growth over the next three years is expected to come from the following:
-
Business growth and expansion among all segments; and
-
Development in each business segment of new products and services that provide increased benefits to patients, healthcare workers and researchers.
Results of Continuing Operations
Medical Segment
Medical revenues in 2007 of $3.4 billion increased $314 million, or 10%, over 2006, which includes an estimated impact of unfavorable foreign currency translation of 3 percentage points.
The following is a summary of revenues by organizational unit:
| Estimated | |||||||||||
| Foreign | |||||||||||
|
Total
|
Exchange | ||||||||||
| (millions of dollars) | 2007 | 2006 |
Change
|
Impact | |||||||
| Medical Surgical Systems | $ | 1,864 | $ | 1,749 | 7% | 2% | |||||
| Diabetes Care | 696 | 657 | 6% | 2% | |||||||
| Pharmaceutical Systems | 792 | 640 | 24% | 6% | |||||||
| Ophthalmic Systems | 69 | 62 | 11% | 4% | |||||||
| Total Revenues* | $ | 3,421 | $ | 3,107 | 10% | 3% | |||||
| * Amounts may not add due to rounding. |
Medical revenues reflect the growth of the Pharmaceutical Systems unit and the continued global conversion to safety-engineered products. The Pharmaceutical Systems unit grew by 24%, reflecting the increased use of prefillable syringes by pharmaceutical companies to market new vaccines and bio-tech drugs, especially in the United States. Revenue growth in the Medical Surgical Systems unit was primarily driven by the growth in safety-engineered products and prefilled flush syringes. Sales of safety-engineered products increased 6% in the United States and 30% internationally. For 2008, we expect the full-year revenue growth for the Medical Segment to be about 8%.
Medical operating income was $972 million, or 28.4% of Medical revenues, in 2007, as compared with $864 million, or 27.8% in 2006. The increase in operating income as a percentage of revenues reflects gross margin improvement from increased sales of products that have higher overall gross profit margins, in particular, safety-engineered products and pen needles, as well as favorable manufacturing efficiencies associated with higher volumes and increased leverage on selling and administrative expenses. These improvements were slightly offset by manufacturing start-up costs. See further discussion on gross profit margin below. Selling and administrative expense as a percent of Medical revenues in 2007 declined to 18.9% of revenues from 19.6% of revenues in 2006, primarily due to tight expense controls over base spending. Research and development expenses in 2007 increased $16 million, or 17%, reflecting continued investment in the development of new products and platforms, and included investments in additional resources to enhance our product development process.
Diagnostics Segment
Diagnostics revenues in 2007 of $1.9 billion increased $190 million, or 11%, over 2006, which reflected an estimated favorable impact of foreign currency translation of about 2 percentage points.
The following is a summary of revenues by organizational unit:
| Estimated | |||||||||||
| Foreign | |||||||||||
| Total | Exchange | ||||||||||
| (millions of dollars) | 2007 | 2006 |
Change
|
Impact | |||||||
| Preanalytical Systems | $ | 1,007 | $ | 928 | 9% | 3% | |||||
| Diagnostic Systems | 898 | 787 | 14% | 2% | |||||||
| Total Revenues | $ | 1,905 | $ | 1,715 | 11% | 2% |
21
Financial Review Becton, Dickinson and Company
Revenue growth in the Preanalytical Systems unit was driven by the continued conversion to safety-engineered products, which accounted for sales of $718 million as compared with $627 million in the prior year. Sales of safety-engineered products reflected growth of 9% in the United States, which benefited from BD Vacutainer Push Button Blood Collection Set conversion activity, and 25% internationally. The Diagnostics Systems unit experienced solid worldwide sales of its automated diagnostic platforms, including the molecular BD ProbeTec and BD Viper systems, along with solid growth of its BD BACTEC blood culture and TB systems and the BD Phoenix ID/AST platform. Those platforms reported combined incremental sales of $35 million over 2006. In addition, the Diagnostic Systems revenue growth includes $88 million of revenues from TriPath and $13 million of incremental revenues from GeneOhm. Sales of flu diagnostic tests declined $36 million in fiscal 2007 compared with 2006, primarily due to relatively mild flu seasons in both the United States and Japan and the termination of our supply arrangement with our Japanese supplier. For 2008, we expect full year revenue growth for the Diagnostics Segment to be about 9%.
Diagnostics operating income was $343 million, or 18.0% of Diagnostics revenues in 2007, compared with $390 million, or 22.8% in 2006. Segment operating income reflects the in-process research and development charges of $115 million in 2007 related to the TriPath acquisition and $53 million in 2006 related to the GeneOhm acquisition. The Diagnostics Segment experienced a slight improvement in gross profit margin from sales growth of products that have higher overall gross profit margins, in particular, safety-engineered products and the BD ProbeTec system. These improvements were slightly offset by manufacturing start-up costs. See further discussion on gross profit margin below. Selling and administrative expense as a percent of Diagnostics revenues in 2007 was higher than the comparable amount in 2006 primarily due to the impact of TriPath and GeneOhm. Research and development expense increased $33 million, or 39%, reflecting new spending associated with these two acquisitions and overall increased investment in new product development.
Biosciences Segment
Biosciences revenues in 2007 of $1.0 billion increased $118 million, or 13%, over 2006, which reflected an estimated impact of favorable foreign currency translation of 3 percentage points.
The following is a summary of revenues by organizational unit:
| Estimated | |||||||||||
| Foreign | |||||||||||
|
Total
|
Exchange | ||||||||||
| (millions of dollars) | 2007 | 2006 |
Change
|
Impact | |||||||
| Immunocytometry Systems | $ | 588 | $ | 503 | 17% | 3% | |||||
| Discovery Labware | 278 | 256 | 9% | 2% | |||||||
| Pharmingen | 168 | 157 | 7% | 2% | |||||||
| Total Revenues | $ | 1,034 | $ | 916 | 13% | 3% |
Revenue growth in the Immunocytometry Systems unit reflects strong sales of instruments and flow cytometry reagents, driven by increased demand for research analyzers and clinical reagents. Revenue growth in the Discovery Labware unit reflects strong sales of bionutrients and overall market growth. For 2008, we expect the full year revenue growth for the Biosciences Segment to be about 8 to 9%.
Biosciences operating income was $259 million, or 25.0% of Biosciences revenues in 2007, compared with $222 million, or 24.2% in 2006. Segment operating income includes an in-process research and development charge of $7 million in 2007. The increase in operating income, as a percentage of revenues, reflects gross profit improvement from relatively higher sales growth of products that have higher overall gross profit margins and the favorable impact of foreign currency translation. These improvements were offset by manufacturing start-up costs. See further discussion on gross profit margin below. Selling and administrative expense as a percentage of Biosciences revenues was 24.0% versus 25.3% in 2006. Higher sales and continued tight expense control were the key contributors to the increased expense leverage. Research and development expense in 2007 increased $7 million, or 9.0%, reflecting spending on new product development and advanced technology, particularly in the Immunocytometry Systems unit.
22
Financial Review Becton, Dickinson and Company
Geographic Revenues
Revenues in the United States in 2007 of $3.0 billion increased 11%. U.S. sales of safety-engineered devices were approximately $982 million in 2007, compared with $917 million in 2006. Growth was also led by strong sales of prefilled flush syringes, prefillable syringes and immunocytometry instruments and reagents. U.S. revenue growth also included $88 million of revenues from TriPath.
Revenues outside the United States in 2007 increased 11% to $3.3 billion, reflecting an estimated impact of favorable foreign currency translation of 5 percentage points. Growth was led by solid sales in our European, Asia Pacific and Canadian regions in 2007. International sales of safety-engineered devices were approximately $409 million in 2007, compared with $324 million in 2006.
Gross Profit Margin
Gross profit margin was 51.7% in 2007, compared with 51.3% in 2006. Gross profit margin in the current year as compared with the prior year reflected an estimated 0.6% improvement relating to increased sales of products with relatively higher margins as well as productivity gains. These improvements were partially offset by an estimated 0.2% impact from manufacturing start-up costs. We expect gross profit margin in 2008 to be about the same as in 2007. Expected improvements are anticipated to be offset by increased resin and steel costs as well as manufacturing start-up costs in 2008.
Operating Expenses
Selling and administrative expense was $1.6 billion in 2007 compared with $1.4 billion in 2006, or 25.2% of revenues in both years. Aggregate expenses for 2007 reflect base spending increases of $62 million and expenses of $40 million associated with the GeneOhm and TriPath operations. Increases in selling and administrative expense in 2007 also reflected the absence of proceeds from insurance settlements of $17 million received in the prior year in connection with our previously-owned latex glove business, as well as an unfavorable foreign exchange impact of $35 million. Selling and administrative expense as a percentage of revenues is expected to decrease, on a reported basis, by about 70 basis points for 2008.
Research and development (R&D) expense in 2007 was $360 million, or 5.7% of revenues, compared with $302 million, or 5.3% of revenues, in 2006. The increase in R&D expenditures includes spending for new programs in each of our segments, as previously discussed. R&D expense is expected to increase about 11% for 2008.
Operating Income
Operating margin in 2007 was 18.9% of revenues, compared with 19.9% in 2006. Operating income of $1.2 billion in 2007 reflected $122 million of acquired in-process R&D charges, as further discussed above. Operating income of $1.1 billion in 2006 included $53 million of acquired in-process R&D charges, partially offset by $17 million of insurance settlement proceeds, as discussed above. We expect operating margin to increase 240 to 250 basis points, with 190 basis points attributable to the acquired in-process R&D charges in 2007.
Non-Operating Expense and Income
Interest expense was $46 million in 2007, compared with $66 million in 2006. The decrease reflected lower debt and higher levels of capitalized interest. Interest income was $46 million in 2007, compared with $59 million in 2006, resulting from lower cash balances.
Income Taxes
The effective tax rate in 2007 was 28.9% compared with the prior years rate of 27.6% . The 2007 rate reflected the non-deductibility of the acquired in-process R&D charges of $122 million, which were partially offset by the impact of approximately 0.3% resulting from the retroactive reinstatement of the research and experimentation tax credit. The 2006 rate reflected the non-deductibility of the acquired in-process R&D charge of $53 million, as well as the impact relating to the proceeds received from insurance settlements of approximately 0.2% . In 2008, we expect our effective tax rate to be about 27%.
Income and Diluted Earnings per Share from Continuing Operations
Income from continuing operations and diluted earnings per share from continuing operations in 2007 were $856 million and $3.36, respectively. The acquired in-process R&D charges decreased income from continuing operations and diluted earnings per share from continuing operations in the aggregate by $122 million and by $.48, respectively, in 2007. Income from continuing operations and diluted earnings per share from continuing operations in 2006 were $815 million and $3.18, respectively. The acquired in-process R&D charge decreased income from continuing operations and diluted earnings per share from continuing operations by $53 million and by $.21, respectively, in 2006.
23
Financial Review Becton, Dickinson and Company
Discontinued Operations
In September 2006, the Company announced a plan to exit the blood glucose monitoring market. The Company recorded a pre-tax charge of $63 million in connection with its decision to exit the BGM product line. During the first quarter of 2007, the Company received an unsolicited offer for the purchase of the BGM product line. In December 2006, the Company sold the product line for $20 million. Following the sale, prior period Consolidated Statements of Income and Cash Flows were restated to separately present the results of the BGM product line as discontinued operations. The September 30, 2006 Consolidated Balance Sheet was not restated. See Note 3 of the Notes to Consolidated Financial Statements for additional discussion.
Financial Instrument Market Risk
We selectively use financial instruments to manage the impact of foreign exchange rate and interest rate fluctuations on earnings. The counterparties to these contracts are highly rated financial institutions. We do not enter into financial instruments for trading or speculative purposes.
We have foreign currency exposures throughout Europe, Asia Pacific, Canada, Japan and Latin America. We face transactional currency exposures that arise when we enter into transactions in non-hyperinflationary countries, generally on an intercompany basis, that are denominated in currencies other than our functional currency. We hedge substantially all such foreign exchange exposures primarily through the use of forward contracts and currency options. We also face currency exposure that arises from translating the results of our worldwide operations to the U.S. dollar at exchange rates that have fluctuated from the beginning of a reporting period. To partially protect against adverse foreign exchange rate movements, we purchase option and forward contracts to hedge certain forecasted sales that are denominated in foreign currencies. Gains or losses on our derivative instruments are largely offset by the gains or losses on the underlying hedged transactions. For foreign currency derivative instruments, market risk is determined by calculating the impact on fair value of an assumed change in foreign exchange rates relative to the U.S. dollar. Fair values were estimated based on market prices, when available, or dealer quotes. The reduction in fair value of our purchased option contracts is limited to the options fair value. With respect to the derivative instruments outstanding at September 30, 2007, a 10% appreciation of the U.S. dollar over a one-year period would increase pre-tax earnings by $52 million, while a 10% depreciation of the U.S. dollar would decrease pre-tax earnings by $10 million.
Comparatively, considering our derivative instruments outstanding at September 30, 2006, a 10% appreciation of the U.S. dollar over a one-year period would have increased pre-tax earnings by $68 million, while a 10% depreciation of the U.S. dollar would have decreased pre-tax earnings by $3 million. These calculations do not reflect the impact of exchange gains or losses on the underlying positions that would substantially offset the results of the derivative instruments.
Our primary interest rate exposure results from changes in short-term U.S. dollar interest rates. Our debt and interest-bearing investments at September 30, 2007, are substantially all U.S. dollar-denominated. Therefore, transaction and translation exposure relating to such instruments is minimal. When managing interest rate exposures, we strive to achieve an acceptable balance between fixed and floating rate instruments. We may enter into interest rate swaps to help maintain this balance and manage debt and interest-bearing investments in tandem, since these items have an offsetting impact on interest rate exposure. For interest rate derivative instruments, market risk is determined by calculating the impact to fair value of an assumed change in interest rates across all maturities. Fair values are estimated based on dealer quotes. A change in interest rates on short-term debt and interest-bearing investments is assumed to impact earnings and cash flow, but not fair value because of the short maturities of these instruments. A change in interest rates on long-term debt is assumed to impact fair value but not earnings or cash flow because the interest on such obligations is fixed. Based on our overall interest rate exposure at September 30, 2007 and 2006, a change of 10% in interest rates would not have a material effect on our earnings or cash flows over a one-year period. An increase of 10% in interest rates would decrease the fair value of our long-term debt and interest rate swaps at September 30, 2007 and 2006 by approximately $37 million and $39 million, respectively. A 10% decrease in interest rates would increase the fair value of our long-term debt and interest rate swaps at September 30, 2007 and 2006 by approximately $41 million and $33 million, respectively.
24
Financial Review Becton, Dickinson and Company
Liquidity and Capital Resources
Net Cash Flows from Continuing Operating Activities
Net cash provided by continuing operating activities, which continues to be our primary source of funds to finance operating needs and capital expenditures, was $1.2 billion in 2007, compared with $1.1 billion in 2006.
Net Cash Flows from Continuing Investing Activities
Net cash used for continuing investing activities in 2007 was $1.0 billion, compared with $784 million in 2006. Acquisitions of businesses of $340 million in 2007 represented the net cash paid for the TriPath acquisition. Capital expenditures were $556 million in 2007, compared with $457 million in 2006. Medical capital spending of $353 million and Diagnostics capital spending of $114 million in 2007 related primarily to various capacity expansions. Biosciences capital spending of $73 million in 2007 included spending on manufacturing capacity expansions. In 2008, capital expenditures are expected to be in the $600 to $650 million range, reflecting investments in various manufacturing capacity and facility expansions.
Net Cash Flows from Continuing Financing Activities
Net cash used for financing activities was $726 million in 2007, as compared with $342 million in 2006, and included the repurchase of shares of our common stock for approximately $450 million, compared with approximately $449 million in 2006. At September 30, 2007, approximately 11.1 million common shares remained available for purchase, consisting of 1.1 million shares remaining under a November 2005 Board of Directors authorization to repurchase up to 10 million common shares, plus an additional 10 million shares that were authorized for repurchase by the Board of Directors in July 2007. For 2008, we expect that cash used to repurchase common shares will be about $450 million. Total debt at September 30, 2007, was $1.2 billion compared with $1.4 billion at September 30, 2006. Short-term debt decreased to 18% of total debt at year-end, from 31% at the end of 2006. Floating rate debt was 36% of total debt at the end of 2007 and 46% at the end of 2006. Our weighted average cost of total debt at the end of 2007 was 5.7%, up from 5.5% at the end of 2006. Debt-to-capitalization at year-end improved to 20.9% from 25.8% last year.
We have in place a commercial paper borrowing program that is available to meet our short-term financing needs, including working capital requirements. Borrowings outstanding under this program were $200 million at September 30, 2007. We maintain a $1.0 billion syndicated credit facility in order to provide backup support for our commercial paper program and for other general corporate purposes. This credit facility expires in December 2012 and includes a single financial covenant that requires BD to maintain an interest expense coverage ratio (ratio of earnings before income taxes, depreciation and amortization to interest expense) of not less than 5-to-1 for the most recent four consecutive fiscal quarters. On the last eight measurement dates, this ratio had ranged from 17-to-1 to 23-to-1. There were no borrowings outstanding under this facility at September 30, 2007. In addition, we have informal lines of credit outside the United States.
At September 30, 2007, our long-term debt was rated A2 by Moodys and A+ by Standard and Poors, and our commercial paper ratings were P-1 by Moodys and A-1 by Standard and Poors. Given the availability of the various credit facilities and our strong credit ratings, we continue to have a high degree of confidence in our ability to refinance maturing short-term and long-term debt, as well as to incur substantial additional debt, if required.
BDs ability to generate cash flow from operations, issue debt, enter into other financing arrangements and attract long-term capital on acceptable terms could be adversely affected in the event there was a material decline in the demand for BDs products, deterioration in BDs key financial ratios or credit ratings or other significantly unfavorable changes in conditions. While a deterioration in the Companys credit ratings would increase the costs associated with maintaining and borrowing under its existing credit arrangements, such a downgrade would not affect the Companys ability to draw on these credit facilities, nor would it result in an acceleration of the scheduled maturities of any outstanding debt.
25
Financial Review Becton, Dickinson and Company
Contractual Obligations
In the normal course of business, we enter into contracts and commitments that obligate us to make payments in the future. The table below sets forth BDs significant contractual obligations and related scheduled payments:
| 2009 to | 2011 to | 2013 and | ||||||||||||||
| (millions of dollars) | Total | 2008 | 2010 | 2012 | Thereafter | |||||||||||
| Short-term debt | $ | 208 | $ | 208 | $ | | $ | | $ | | ||||||
| Long-term debt(A) | 1,594 | 54 | 301 | 85 | 1,154 | |||||||||||
| Operating leases | 153 | 46 | 59 | 32 | 16 | |||||||||||
| Purchase obligations(B) | 365 | 296 | 50 | 19 | | |||||||||||
| Total(C) | $ | 2,320 | $ | 604 | $ | 410 | $ | 136 | $ | 1,170 |
| (A) |
Long-term debt obligations include expected principal and interest obligations, including interest rate swaps. The interest rate forward curve at September 30, 2007 was used to compute the amount of the contractual obligation for variable rate debt instruments and swaps. |
| (B) |
Purchase obligations are for purchases made in the normal course of business to meet operational and capital requirements. |
| (C) |
Required funding obligations for 2008 relating to pension and other postretirement benefit plans are not expected to be material. |
2006 Compared With 2005
Worldwide revenues in 2006 of $5.7 billion increased 7% from 2005 and reflected estimated volume increases of 7%, an estimated decrease due to unfavorable foreign currency translation of 1%, and estimated price increases of less than 1%.
Medical Segment
Medical revenues in 2006 of $3.1 billion increased $222 million, or 8%, over 2005.
The following is a summary of revenues by organizational unit:
| Estimated | |||||||||||
| Foreign | |||||||||||
| Total | Exchange | ||||||||||
| (millions of dollars) | 2006 | 2005 | Change* | Impact | |||||||
| Medical Surgical Systems | $ | 1,749 | $ | 1,661 | 5% | | |||||
| Diabetes Care | 657 | 600 | 9% | (1% | ) | ||||||
| Pharmaceutical Systems | 640 | 563 | 14% | (3% | ) | ||||||
| Ophthalmic Systems | 62 | 60 | 3% | (2% | ) | ||||||
| Total Revenues* | $ | 3,107 | $ | 2,884 | 8% | (1% | ) | ||||
| * Amounts may not calculate due to rounding. | |||||||||||
Medical revenue growth was driven by the continued conversion to safety-engineered products, which accounted for sales of $613 million, as compared with $571 million in the prior year, reflecting growth of 6% in the United States and 16% internationally. Revenue growth in the Medical Surgical Systems unit of this segment was primarily driven by the growth in safety-engineered products and prefilled flush syringes. Revenue growth in the Pharmaceutical Systems unit was driven by a 26% increase in sales in the United States. The Diabetes Care units revenue growth reflected strong sales of pen needles worldwide.
Medical operating income was $864 million, or 27.8% of Medical revenues, in 2006, as compared with $748 million, or 25.9% in 2005. The Segments gross profit margin in 2006 reflected improvement associated with relatively higher sales growth of products that have higher overall gross profit margins, in particular, safety-engineered products and pen needles, as well as favorable manufacturing efficiencies associated with higher volumes. See further discussion on gross profit margin below. Selling and administrative expense as a percent of Medical revenues in 2006 was slightly lower compared with 2005, primarily due to tight expense controls over base spending. Research and development expense in 2006 increased $7 million, or 8%, reflecting continued investment in the development of new products and platforms.
Diagnostics Segment
Diagnostics revenues in 2006 of $1.7 billion increased $83 million, or 5%, over 2005, which reflected an estimated unfavorable impact of foreign currency translation of about 1 percentage point.
The following is a summary of revenues by organizational unit:
| Estimated | |||||||||||
| Foreign | |||||||||||
| Total | Exchange | ||||||||||
| (millions of dollars) | 2006 | 2005 | Change | Impact | |||||||
| Preanalytical Systems | $ | 928 | $ | 855 | 9 | % | | ||||
| Diagnostic Systems | 787 | 777 | 1 | % | (1 | %) | |||||
| Total Revenues | $ | 1,715 | $ | 1,632 | 5 | % | (1 | %) |
26
Financial Review Becton, Dickinson and Company
Revenue growth in the Preanalytical Systems unit was driven by the continued conversion to safety-engineered products, which accounted for sales of $627 million, as compared with $543 million in 2005. Sales of safety-engineered products reflected growth of 13% in the United States, which benefited from BD Vacutainer Push Button Blood Collection Set conversion activity, and 20% internationally. The Diagnostic Systems unit experienced solid worldwide sales of its automated diagnostic platforms, including the molecular BD ProbeTec ET, BD BACTEC, and the BD Phoenix ID/AST. These platforms reported combined incremental sales of $33 million over 2005. Revenues for GeneOhm, which was acquired in February 2006, totaled $8 million. Sales of flu diagnostic tests declined by approximately $11 million in fiscal 2006 compared with 2005, primarily due to a relatively mild flu season in both Japan and the United States.
Diagnostics operating income was $390 million, or 22.8% of Diagnostics revenues, in 2006, compared with $403 million, or 24.7%, in 2005. Segment operating income for 2006 reflects the acquired in-process research and development charge of $53 million as well as the operating results of GeneOhm, which in the aggregate, reduced operating income as a percentage of Diagnostics revenues by approximately 5%. The Diagnostics Segment experienced slight gross profit margin improvement reflecting higher prices and productivity, which was substantially offset by the impact of the recently acquired GeneOhm products, which have lower overall gross profit margins, and lower sales growth of flu diagnostic products, which have higher overall gross profit margins. See further discussion on gross profit margin below. Selling and administrative expense as a percent of Diagnostics revenues in 2006 was lower compared with 2005 primarily due to tight controls on spending, which more than offset the incremental GeneOhm expenses. Research and development expense in 2006 increased $6 million, or 7%, reflecting new spending for product development associated with the GeneOhm acquisition.
Biosciences Segment
Biosciences revenues in 2006 of $916 million increased $92 million, or 11%, over 2005, which reflected an estimated impact of unfavorable foreign currency translation of 1 percentage point.
The following is a summary of revenues by organizational unit:
| Estimated | ||||||||||||
| Foreign | ||||||||||||
| Total | Exchange | |||||||||||
| (millions of dollars) | 2006 | 2005 | Change* | Impact | ||||||||
| Immunocytometry Systems | $ | 503 | $ | 452 | 11 | % | (1 | %) | ||||
| Discovery Labware | 256 | 231 | 11 | % | (1 | %) | ||||||
| Pharmingen | 157 | 141 | 12 | % | (1 | %) | ||||||
| Total Revenues | $ | 916 | $ | 824 | 11 | % | (1 | %) | ||||
| * Amounts may not calculate due to rounding. | ||||||||||||
Revenue growth in the Immunocytometry Systems unit reflected strong sales of instruments and flow cytometry reagents, driven by increased demand for research and clinical analyzers. Revenue growth rates in the Immunocytometry Systems and Pharmingen units were favorably impacted by the adverse effect a cancellation of a distribution agreement had on revenues in 2005. As a result of an inventory repurchase obligation to this distributor upon termination of the arrangement, certain sales made to this distributor in the latter part of 2005 ($5 million in Immunocytometry Systems and $12 million in Pharmingen) were not recognized as revenue. In addition, sales in 2006 were favorably impacted by higher average selling prices as a result of terminating the arrangement. Revenue growth in the Discovery Labware unit resulted primarily from strong sales of bionutrients and market share gains.
Biosciences operating income was $222 million, or 24.2% of Biosciences revenues in 2006, compared with $186 million, or 22.5% in 2005. The increase in operating income, as a percentage of revenues, reflects gross profit improvement from the favorable impact of terminating a distribution agreement in 2005, increased operating efficiencies, as well as relatively higher sales growth of products that have higher overall gross profit margins. See further discussion on gross profit margin below. Selling and administrative expense as a percent of Biosciences revenues was lower compared with 2005, primarily due to higher revenues and the absence of $8 million of costs incurred in 2005 associated with the termination of the distribution agreement, mentioned above. Research and development expense in 2006 increased $8 million, or 13%, reflecting spending on new product development and advanced technology, particularly in the Immunocytometry Systems unit and bioimaging products.
27
Financial Review Becton, Dickinson and Company
Geographic Revenues
Revenues in the United States in 2006 of $2.7 billion increased 9%. U.S. sales of safety-engineered devices were approximately $917 million in 2006, compared with $842 million in 2005. Growth was also led by strong sales of diabetes care products, prefilled flush syringes and prefillable syringes. Revenues of immunocytometry instruments and reagents also demonstrated good growth.
Revenues outside the United States in 2006 increased 6% to $3 billion, reflecting an estimated impact of unfavorable foreign currency translation of 2 percentage points. Growth was led by strong sales in our Asia Pacific, Canadian and European regions in 2006. International sales of safety-engineered devices were approximately $324 million in 2006, compared with $273 million in 2005.
Gross Profit Margin
Gross profit margin was 51.3% in 2006, compared with 50.9% in 2005. Gross profit margin in 2006 reflected an estimated 1.0% improvement relating to increased sales growth of products with relatively higher margins and to productivity gains. These improvements were partially offset by an estimated 0.2% impact from foreign currency translation, an estimated 0.3% unfavorable impact of higher raw material costs and 0.1% relating to an increase in share-based compensation.
Operating Expenses
Selling and administrative expense of $1.4 billion in 2006 was 25.2% of revenues, compared with $1.4 billion or 26.0% of revenues in 2005. Aggregate expenses for 2006 reflect base spending increases of $49 million and expenses associated with recent acquisitions, primarily GeneOhm, of $17 million. Selling and administrative expense in 2006 also reflected increases primarily in share-based compensation expense of $25 million. These increases were partially offset by a favorable foreign exchange impact of $13 million and by proceeds from insurance settlements of $17 million received in connection with our previously-owned latex glove business.
Research and development expense in 2006 was $302 million, or 5.3% of revenues, compared with $268 million, or 5.0% of revenues, in 2005. The increase in R&D expenditures reflected spending for new programs in each of our segments, as previously discussed.
Non-Operating Expense and Income
Interest expense was $66 million in 2006, compared with $56 million in 2005. The increase reflected higher debt levels and the impact of higher interest rates on floating rate debt and on fixed-to-floating interest rate swap transactions. Such swap transactions consist of fair value hedges of certain fixed-rate instruments under which the difference between fixed and floating interest rates is exchanged at specified intervals. Interest income was $59 million in 2006, compared with $36 million in 2005, and reflected higher interest rates and cash balances.
Income Taxes
The effective tax rate in 2006 was 27.6% and reflected the unfavorable impact of the non-deductibility of the acquired in-process R&D charge. The effective tax rate in 2005 was 31.3% and reflected a 7.7% increase relating to the charge in 2005 attributable to the planned repatriation of earnings in 2006 under the American Jobs Creation Act of 2004. In addition, the effective tax rate in 2005 reflected a 1.0% benefit due to the reversal of tax accruals in connection with the conclusion of tax examinations in four non-U.S. jurisdictions.
Income and Diluted Earnings per Share from Continuing Operations
Income from continuing operations and diluted earnings per share from continuing operations in 2006 were $815 million and $3.18, respectively. The in-process R&D charge decreased income from continuing operations and diluted earnings per share from continuing operations by $53 million and by $.21 in 2006. Income from continuing operations and diluted earnings per share from continuing operations in 2005 were $713 million and $2.73, respectively. The tax repatriation charge decreased income from continuing operations by $77 million and diluted earnings per share from continuing operations by $.30 in 2005.
28
Financial Review Becton, Dickinson and Company
Liquidity and Capital Resources
Net Cash Flows from Continuing Operating Activities
Net cash provided by continuing operating activities was $1.1 billion in 2006, reduced from $1.2 billion in 2005, reflecting higher inventory levels and higher income tax payments, including taxes associated with the repatriation of earnings, as discussed further below.
Net Cash Flows from Continuing Investing Activities
Net cash used for continuing investing activities in 2006 was $784 million, compared with $380 million in 2005. Acquisitions of businesses of $231 million in 2006 represented the net cash paid for the GeneOhm acquisition. Capital expenditures were $457 million in 2006, compared with $316 million in 2005. Medical capital spending of $269 million and Diagnostics capital spending of $105 million related primarily to various capacity expansions. Biosciences capital spending of $39 million included spending on manufacturing capacity expansions.
Net Cash Flows from Continuing Financing Activities
Net cash used for financing activities was $342 million in 2006, as compared with $516 million in 2005, and included the repurchase of shares of our common stock for approximately $449 million, compared with approximately $550 million in 2005. Total debt at September 30, 2006, was $1.4 billion compared with $1.3 billion at September 30, 2005. Short-term debt increased to 31% of total debt at year-end, from 16% at the end of 2005. Floating rate debt was 46% of total debt at the end of 2006 and 41% at the end of 2005. Our weighted average cost of total debt at the end of 2006 was 5.5%, up from 5.3% at the end of 2005, due to higher short-term interest rates. Debt-to-capitalization at year-end improved to 25.8% in 2006 from 27.1% in 2005.
The American Jobs Creation Act of 2004 (the AJCA) was signed into law in October 2004. The AJCA creates a temporary incentive for U.S. multinationals to repatriate accumulated income earned outside the United States. As a result of the passage of the AJCA, we repatriated approximately $1.3 billion in 2006 in accordance with our planned repatriation under the AJCA. Uses of the repatriated funds include cash expenditures for compensation and benefits to existing and newly hired U.S. workers, U.S. infrastructure and capital investments and other activities as permitted under the AJCA.
Critical Accounting Policies
The preparation of the consolidated financial statements requires management to use estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, as well as the disclosure of contingent assets and liabilities at the date of the consolidated financial statements. Some of those judgments can be subjective and complex and, consequently, actual results could differ from those estimates. Management bases its estimates and judgments on historical experience and on various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. For any given estimate or assumption made by management, it is possible that other people applying reasonable judgment to the same facts and circumstances could develop different estimates. Actual results that differ from managements estimates could have an unfavorable effect on our consolidated financial statements. Management believes the following critical accounting policies reflect the more significant judgments and estimates used in the preparation of the consolidated financial statements.
Revenue Recognition
Revenue from product sales is recognized when title and risk of loss pass to the customer. We recognize revenue for certain instruments sold from the Biosciences segment upon installation at a customers site. Based upon terms of the sales agreements, the Biosciences segment recognizes revenue in accordance with Emerging Issues Task Force No. 00-21, Revenue Arrangements with Multiple Deliverables. These sales agreements have multiple deliverables, and as such are divided into separate units of accounting. Revenue is recognized upon the completion of each deliverable based on the relative fair values of items delivered.
BDs domestic businesses sell products primarily to distributors who resell the products to end-user customers. We provide rebates to distributors that sell to end-user customers at prices determined under a contract between BD and the end-user customer. Provisions for rebates, as well as sales discounts and returns, are accounted for as a reduction of revenues when revenue is recognized.
29
Financial Review Becton, Dickinson and Company
Impairment of Assets
Pursuant to SFAS No. 142, Goodwill and Other Intangible Assets, goodwill and indefinite-lived intangible assets are subject to impairment reviews at least annually, or whenever indicators of impairment arise. Intangible assets other than goodwill and indefinite-lived intangible assets and other long-lived assets are reviewed for impairment in accordance with SFAS No. 144, Accounting for the Impairment or Disposal of Long-Lived Assets. Impairment reviews are based on a cash flow approach that requires significant management judgment with respect to future volume, revenue and expense growth rates, changes in working capital use, appropriate discount rates and other assumptions and estimates. The estimates and assumptions used are consistent with BDs business plans. The use of alternative estimates and assumptions could increase or decrease the estimated fair value of the asset, and potentially result in different impacts to BDs results of operations. Actual results may differ from managements estimates.
Investments
We hold equity interests in companies having operations or technology in areas within or adjacent to BDs strategic focus. For some of these companies that are publicly traded, market prices are available. However, for those companies that are not publicly traded, fair value is difficult to determine. We write down an investment when management believes an investment has experienced a decline in value that is other than temporary. Future adverse changes in market conditions or poor operating results of the underlying investments could result in an inability to recover the carrying value of the investments, thereby possibly requiring impairment charges in the future.
Tax Valuation Allowances
BD maintains valuation allowances where it is more likely than not that all or a portion of a deferred tax asset will not be realized. Changes in valuation allowances are included in our tax provision in the period of change. In determining whether a valuation allowance is warranted, management evaluates factors such as prior earnings history, expected future earnings, carryback and carryforward periods, and tax strategies that could potentially enhance the likelihood of realization of a deferred tax asset.
Contingencies
We are involved, both as a plaintiff and a defendant, in various legal proceedings that arise in the ordinary course of business, including, without limitation, product liability, antitrust, and environmental matters, as further discussed in Note 12 of the Notes to Consolidated Financial Statements. We assess the likelihood of any adverse judgments or outcomes to these matters as well as potential ranges of probable losses. In accordance with U.S. generally accepted accounting principles, we establish accruals to the extent probable future losses are estimable (in the case of environmental matters, without considering possible third-party recoveries). A determination of the amount of accruals, if any, for these contingencies is made after careful analysis of each individual issue and, when appropriate, is developed after consultation with outside counsel. The accruals may change in the future due to new developments in each matter or changes in our strategy in dealing with these matters.
Given the uncertain nature of litigation generally, we are not able in all cases to estimate the amount or range of loss that could result from an unfavorable outcome of the litigation to which we are a party. In view of these uncertainties, we could incur charges in excess of any currently established accruals and, to the extent available, excess liability insurance. Accordingly, in the opinion of management, any such future charges, individually or in the aggregate, could have a material adverse effect on BDs consolidated results of operations and consolidated net cash flows.
Benefit Plans
We have significant net pension and postretirement benefit costs that are measured using actuarial valuations. Inherent in these valuations are key assumptions including discount rates and expected return on plan assets. We evaluate these key assumptions at least annually on a plan- and country-specific basis. We consider current market conditions, including changes in interest rates and market returns, in selecting these assumptions. Changes in the related net pension and post-retirement benefits costs may occur in the future due to changes in assumptions.
The discount rate is selected to reflect the prevailing rate on September 30 based on investment grade bonds and other factors. Specifically, for the U.S. pension plan, we use an actuarially-determined yield curve to determine the discount rate. We increased our discount rate for the U.S. pension and postretirement plans at September 30, 2007 from 5.95% to 6.35% and increased the rate at September 30, 2006 from 5.5% to 5.95%.
30
Financial Review Becton, Dickinson and Company
To determine the expected long-term rate of return on pension plan assets, we consider the historical and expected returns on various plan asset classes, as well as current and expected asset allocations. At September 30, 2007, the one-year rate of return on assets for our U.S. pension plans was 14.6%, the five-year rate of return was 13.0%, and the ten-year rate of return was 6.5% . We believe that these results, in connection with our current and expected asset allocation, support our assumed long-term return of 8.0% on those assets.
Sensitivity to changes in key assumptions for our U.S. pension and postretirement plans are as follows:
-
Discount rateA change of plus (minus) 25 basis points, with other assumptions held constant, would have an estimated $7 million favorable (unfavorable) impact on the total U.S. net pension and postretirement benefit plan cost.
-
Expected return on plan assetsA change of plus (minus) 25 basis points, with other assumptions held constant, would have an estimated $2 million favorable (unfavorable) impact on U.S. pension plan cost.
Stock-Based Compensation
Compensation cost relating to share-based
payment transactions is recognized in net income using a fair value measurement
method, in accordance with SFAS No. 123(R). SFAS No. 123(R) requires all share-based
payments to employees, including grants of employee stock options, to be recognized
in the statement of operations as compensation expense (based on their fair
values) over the vesting period of the awards. We determine the fair value
of certain share-based awards using a lattice-based binomial option valuation
model that incorporates certain assumptions, such as the risk-free interest
rate, expected volatility, expected dividend yield and expected life of the
options. See Note 13 of the Notes to Consolidated Financial Statements for
additional discussion.
Cautionary Statement Regarding
Forward-Looking Statements
BD and its representatives may from time-to-time make certain forward-looking statements in publicly released materials, both written and oral, including statements contained in this report and filings with the Securities and Exchange Commission and in our other reports to shareholders. Forward-looking statements may be identified by the use of words such as plan, expect, believe, intend, will, anticipate, estimate and other words of similar meaning in conjunction with, among other things, discussions of future operations and financial performance, as well as our strategy for growth, product development, regulatory approvals, market position and expenditures.
All statements that address operating performance or events or developments that we expect or anticipate will occur in the futureincluding statements relating to volume growth, sales and earnings per share growth and statements expressing views about future operating resultsare forward-looking statements.
Forward-looking statements are based on current expectations of future events. The forward-looking statements are, and will be, based on managements then-current views and assumptions regarding future events and operating performance, and speak only as of their dates. Investors should realize that if underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could vary materially from our expectations and projections. Investors are therefore cautioned not to place undue reliance on any forward-looking statements. Furthermore, we undertake no obligation to update or revise any forward-looking statements whether as a result of new information, future events and developments or otherwise.
The following are some important factors that could cause our actual results to differ from our expectations in any forward-looking statements:
-
Regional, national and foreign economic factors, including inflation and fluctuations in interest rates and foreign currency exchange rates and the potential effect of such fluctuations on revenues, expenses and resulting margins, as well as competition in certain markets.
-
We operate in a highly competitive environment. New product introductions by our current or future competitors (for example, new forms of drug delivery) could adversely affect our ability to compete in the global market. Patents attained by competitors, particularly as patents on our products expire, may also adversely impact our competitive position. Certain competitors have established manufactur- ing sites or have contracted with suppliers in low-cost manufacturing locations as a means to lower their costs. New entrants may also appear.
-
Changes in domestic and foreign healthcare industry prac- tices and regulations resulting in increased pricing pressures, including the continued consolidation among healthcare providers; trends toward managed care and healthcare cost containment; and government laws and regulations relating to sales and promotion, reimbursement and pricing generally.
-
The effects, if any, of governmental and media activities regarding the business practices of group purchasing organizations, which negotiate product prices on behalf of their member hospitals with BD and other suppliers.
31
Financial Review Becton, Dickinson and Company
-
Fluctuations in the cost and availability of oil-based resins and other raw materials and the ability to maintain favorable supplier arrangements and relationships (particu- larly with respect to sole-source suppliers) and the potential adverse effects of any disruption in the availability of such raw materials.
-
Our ability to obtain the anticipated benefits of restructuring programs, if any, that we may undertake.
-
Adoption of or changes in government laws and regulations affecting domestic and foreign operations, including those relating to trade, monetary and fiscal policies, taxation, environmental matters, sales practices, price controls, licensing and regulatory approval of new products, regulatory requirements for products in the postmarketing phase, or changes in enforcement practices with respect to any such laws and regulations. In particular, environ- mental laws, particularly with respect to the emission of greenhouse gases, are becoming more stringent throughout the world, which may increase our costs of operations or necessitate changes in our manufacturing plants or processes.
-
Fluctuations in U.S. and international governmental funding and policies for life sciences research.
-
Difficulties inherent in product development, including the potential inability to successfully continue technological innovation, complete clinical trials, obtain regulatory approvals in the United States and abroad, obtain coverage and adequate reimbursement for new products, or gain and maintain market approval of products, as well as the possibility of encountering infringement claims by competitors with respect to patent or other intellectual property rights, all of which can preclude or delay commercialization of a product.
-
Pending and potential litigation or other proceedings adverse to BD, including antitrust claims, product liability claims, patent infringement claims and the availability or collectibility of insurance.
-
The effects, if any, of adverse media exposure or other publicity regarding BDs business or operations.
-
Our ability to achieve earnings forecasts, which are generated based on projected volumes and sales of many product types, some of which are more profitable than others. There can be no assurance that we will achieve any projected level or mix of product sales.
-
The effect of market fluctuations on the value of assets in BDs pension plans and the possibility that BD may need to make additional contributions to the plans as a result of any decline in the value of such assets.
-
Our ability to effect infrastructure enhancements and incorporate new systems technologies into our operations.
-
Product efficacy or safety concerns resulting in product recalls, regulatory action on the part of the U.S. Food and Drug Administration (or foreign counterparts) or declining sales.
-
Economic and political conditions in international markets, including civil unrest, terrorist activity, governmental changes, restrictions on the ability to transfer capital across borders and expropriation of assets by a government.
-
The effects of natural disasters, including hurricanes or pandemic diseases, on our ability to manufacture our products, particularly where production of a product line is concentrated in one or more plants, or on our ability to source components from suppliers that are needed for such manufacturing.
-
Our ability to penetrate developing and emerging markets, which also depends on economic and political conditions, and how well we are able to acquire or form strategic business alliances with local companies and make necessary infrastructure enhancements to production facilities, distribution networks, sales equipment and technology.
-
The impact of business combinations, including acquisitions and divestitures, both internally for BD and externally in the healthcare industry.
-
Issuance of new or revised accounting standards by the Financial Accounting Standards Board or the Securities and Exchange Commission.
The foregoing list sets forth many, but not all, of the factors that could impact our ability to achieve results described in any forward-looking statements. Investors should understand that it is not possible to predict or identify all such factors and should not consider this list to be a complete statement of all potential risks and uncertainties.
32
| Reports of Management | Becton, Dickinson and Company | |
Managements
Responsibilities
The following financial statements have
been prepared by management in conformity with U.S. generally accepted accounting
principles and include, where required, amounts based on the best estimates
and judgments of management. The integrity and objectivity of data in the financial
statements and elsewhere in this Annual Report are the responsibility of management.
In fulfilling its responsibilities for the integrity of the data presented and to safeguard the Companys assets, management employs a system of internal accounting controls designed to provide reasonable assurance, at appropriate cost, that the Companys assets are protected and that transactions are appropriately authorized, recorded and summarized. This system of control is supported by the selection of qualified personnel, by organizational assignments that provide appropriate delegation of authority and division of responsibilities, and by the dissemination of written policies and procedures. This control structure is further reinforced by a program of internal audits, including a policy that requires responsive action by management.
The Board of Directors monitors the internal control system, including internal accounting and financial reporting controls, through its Audit Committee, which consists of six independent Directors. The Audit Committee meets periodically with the independent registered public accounting firm, the internal auditors and management to review the work of each and to satisfy itself that they are properly discharging their responsibilities. The independent registered public accounting firm and the internal auditors have full and free access to the Audit Committee and meet with its members, with and without management present, to discuss the scope and results of their audits including internal control, auditing and financial reporting matters.
Managements Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rule 13a-15(f) under the Securities Act of 1934. Management conducted an assessment of the effectiveness of internal control over financial reporting based on the criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on this assessment and those criteria, management concluded that internal control over financial reporting was effective as of September 30, 2007.
The financial statements and internal control over financial reporting have been audited by Ernst & Young LLP, an independent registered public accounting firm. Ernst & Youngs reports with respect to fairness of presentation of the statements, and the effectiveness of internal control over financial reporting are included herein.
 |
 |
 |
||
| Edward J. Ludwig | John R. Considine | William A. Tozzi | ||
| Chairman, President and | Senior Executive Vice President | Vice President | ||
| Chief Executive Officer | and Chief Financial Officer | Finance |
33
| Report of Independent Registered Public | Becton, Dickinson and Company | |
| Accounting Firm |
To the Shareholders and Board of
Directors of Becton, Dickinson and Company
We have audited the accompanying consolidated balance sheets of Becton, Dickinson and Company as of September 30, 2007 and 2006, and the related consolidated statements of income, comprehensive income, and cash flows for each of the three years in the period ended September 30, 2007. These financial statements are the responsibility of the Companys management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Becton, Dickinson and Company at September 30, 2007 and 2006, and the consolidated results of its operations and its cash flows for each of the three years in the period ended September 30, 2007, in conformity with U.S. generally accepted accounting principles.
As discussed in Notes 2 and 5 to the consolidated financial statements, the Company adopted Financial Accounting Standard No. 158, Employers Accounting for Defined Benefit Pension and Other Postretirement Plans, an amendment of FASB Statements No. 87, 88, 106 and 132(R) on September 30, 2007.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of Becton, Dickinson and Companys internal control over financial reporting as of September 30, 2007, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated November 16, 2007 expressed an unqualified opinion thereon.
ERNST & YOUNG LLP
New York, New York
November 16, 2007
34
| Report of Independent Registered Public | ||
| Accounting Firm |
To the Shareholders and Board of
Directors of Becton, Dickinson and Company
We have audited Becton, Dickinson and Companys internal control over financial reporting as of September 30, 2007, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Becton, Dickinson and Companys management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Managements Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the companys internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A companys internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A companys internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the companys assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Becton, Dickinson and Company maintained, in all material respects, effective internal control over financial reporting as of September 30, 2007, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Becton, Dickinson and Company as of September 30, 2007 and 2006, and the related consolidated statements of income, comprehensive income, and cash flows for each of the three years in the period ended September 30, 2007 of Becton, Dickinson and Company, and our report dated November 16, 2007 expressed an unqualified opinion thereon.
ERNST & YOUNG LLP
New York, New York
November 16, 2007
35
| Financial Statements | Becton, Dickinson and Company | |
Consolidated Statements
of Income
Years Ended September 30
Thousands of dollars, except per share
amounts
| 2007 | 2006 | 2005 | ||||||||||
| Operations | ||||||||||||
| Revenues | $ | 6,359,708 | $ | 5,738,017 | $ | 5,340,833 | ||||||
| Cost of products sold | 3,071,921 | 2,793,265 | 2,622,427 | |||||||||
| Selling and administrative expense | 1,602,404 | 1,448,166 | 1,386,897 | |||||||||
| Research and development expense | 360,050 | 301,872 | 267,664 | |||||||||
| Acquired in-process research and development | 122,133 | 53,300 | | |||||||||
| Total Operating Costs and Expenses | 5,156,508 | 4,596,603 | 4,276,988 | |||||||||
| Operating Income | 1,203,200 | 1,141,414 | 1,063,845 | |||||||||
| Interest expense | (46,420 | ) | (66,046 | ) | (55,673 | ) | ||||||
| Interest income | 46,221 | 59,296 | 36,421 | |||||||||
| Other income (expense), net | 944 | (8,762 | ) | (7,064 | ) | |||||||
| Income From Continuing Operations | ||||||||||||
| Before Income Taxes | 1,203,945 | 1,125,902 | 1,037,529 | |||||||||
| Income tax provision | 347,778 | 310,792 | 325,009 | |||||||||
| Income from Continuing Operations | 856,167 | 815,110 | 712,520 | |||||||||
| Income (loss) from Discontinued Operations | ||||||||||||
| Net of income tax provision (benefit) of | ||||||||||||
| $15,242, $(32,823) and $(26,877) | 33,866 | (62,830 | ) | 9,743 | ||||||||
| Net Income | $ | 890,033 | $ | 752,280 | $ | 722,263 | ||||||
| Basic Earnings per Share | ||||||||||||
| Income from Continuing Operations | $ | 3.50 | $ | 3.30 | $ | 2.83 | ||||||
| Income (loss) from Discontinued Operations | $ | 0.14 | $ | (0.25 | ) | $ | 0.04 | |||||
| Basic Earnings per Share(A) | $ | 3.63 | $ | 3.04 | $ | 2.87 | ||||||
| Diluted Earnings per Share | ||||||||||||
| Income from Continuing Operations | $ | 3.36 | $ | 3.18 | $ | 2.73 | ||||||
| Income (loss) from Discontinued Operations | $ | 0.13 | $ | (0.24 | ) | $ | 0.04 | |||||
| Diluted Earnings per Share(A) | $ | 3.49 | $ | 2.93 | $ | 2.77 | ||||||
| (A) Total per share amounts may not add due to rounding. | ||||||||||||
| See notes to consolidated financial statements | ||||||||||||
36
| Financial Statements Becton, Dickinson and Company | ||
Consolidated Statements of
Comprehensive Income
Years Ended September 30
Thousands of dollars
| 2007 | 2006 | 2005 | ||||||||||
| Net Income | $ | 890,033 | $ | 752,280 | $ | 722,263 | ||||||
| Other Comprehensive Income (Loss), Net of Tax | ||||||||||||
| Foreign currency translation adjustments | 250,411 | 77,396 | (17,742 | ) | ||||||||
| Minimum pension liability adjustment | 3,159 | 77,086 | 4,494 | |||||||||
| Unrealized (loss) gain on investments, net of amounts recognized | (10,643 | ) | 1,212 | (1,112 | ) | |||||||
| Unrealized loss on cash flow hedges, net of amounts realized | (2,596 | ) | (1,307 | ) | (135 | ) | ||||||
| Other Comprehensive Income (Loss), Net of Tax | 240,331 | 154,387 | (14,495 | ) | ||||||||
| Comprehensive Income | $ | 1,130,364 | $ | 906,667 | $ | 707,768 | ||||||
| See notes to consolidated financial statements | ||||||||||||
37
| Financial Statements Becton, Dickinson and Company | ||
Consolidated Balance Sheets
September 30
Thousands of dollars, except per share amounts and numbers
of shares
| 2007 | 2006 | |||||||
| Assets | ||||||||
| Current Assets | ||||||||
| Cash and equivalents | $ | 511,482 | $ | 1,000,289 | ||||
| Short-term investments | 158,040 | 106,386 | ||||||
| Trade receivables, net | 1,083,152 | 885,748 | ||||||
| Inventories | 1,051,959 | 875,738 | ||||||
| Prepaid expenses, deferred taxes and other | 325,933 | 317,092 | ||||||
| Total Current Assets | 3,130,566 | 3,185,253 | ||||||
| Property, Plant and Equipment, Net | 2,497,338 | 2,133,548 | ||||||
| Goodwill | 621,414 | 565,146 | ||||||
| Core and Developed Technology, Net | 374,779 | 244,811 | ||||||
| Other Intangibles, Net | 95,938 | 91,501 | ||||||
| Capitalized Software, Net | 142,738 | 189,355 | ||||||
| Other | 466,592 | 414,911 | ||||||
| Total Assets | $ | 7,329,365 | $ | 6,824,525 | ||||
| Liabilities | ||||||||
| Current Liabilities | ||||||||
| Short-term debt | $ | 207,634 | $ | 427,218 | ||||
| Accounts payable | 266,993 | 243,602 | ||||||
| Accrued expenses | 481,429 | 490,425 | ||||||
| Salaries, wages and related items | 435,854 | 380,478 | ||||||
| Income taxes | 86,899 | 34,606 | ||||||
| Total Current Liabilities | 1,478,809 | 1,576,329 | ||||||
| Long-Term Debt | 955,713 | 956,971 | ||||||
| Long-Term Employee Benefit Obligations | 444,874 | 270,495 | ||||||
| Deferred Income Taxes and Other | 88,012 | 184,526 | ||||||
| Commitments and Contingencies | | | ||||||
| Shareholders Equity | ||||||||
| Common stock$1 par value: authorized640,000,000 shares; | ||||||||
| issued332,662,160 shares in 2007 and 2006 | 332,662 | 332,662 | ||||||
| Capital in excess of par value | 1,125,368 | 873,535 | ||||||
| Retained earnings | 5,995,787 | 5,345,697 | ||||||
| Deferred compensation | 12,205 | 11,134 | ||||||
| Common stock in treasuryat cost88,825,066 shares in 2007 | ||||||||
| and 87,194,060 shares in 2006 | (3,105,893 | ) | (2,698,016 | ) | ||||
| Accumulated other comprehensive income (loss) | 1,828 | (28,808 | ) | |||||
| Total Shareholders Equity | 4,361,957 | 3,836,204 | ||||||
| Total Liabilities and Shareholders Equity | $ | 7,329,365 | $ | 6,824,525 | ||||
| See notes to consolidated financial statements | ||||||||
38
| Financial Statements Becton, Dickinson and Company | ||
Consolidated Statements of
Cash Flows
Years Ended September 30
Thousands of dollars
| 2007 | 2006 | 2005 | ||||||||||
| Operating Activities | ||||||||||||
| Net income | $ | 890,033 | $ | 752,280 | $ | 722,263 | ||||||
| (Income) loss from discontinued operations, net | (33,866 | ) | 62,830 | (9,743 | ) | |||||||
| Income from continuing operations, net | 856,167 | 815,110 | 712,520 | |||||||||
| Adjustments to income from continuing operations to derive net cash | ||||||||||||
| provided by continuing operating activities, net of amounts acquired: | ||||||||||||
| Depreciation and amortization | 441,341 | 402,332 | 382,669 | |||||||||
| Share-based compensation | 107,706 | 108,613 | 70,199 | |||||||||
| Deferred income taxes | (115,489 | ) | (108,285 | ) | 63,769 | |||||||
| Acquired in-process research and development | 122,133 | 53,300 | | |||||||||
| Change in operating assets and liabilities: | ||||||||||||
| Trade receivables, net | (117,048 | ) | (19,977 | ) | (34,332 | ) | ||||||
| Inventories | (126,863 | ) | (99,505 | ) | (57,371 | ) | ||||||
| Prepaid expenses, deferred taxes and other | (24,965 | ) | (122,496 | ) | (897 | ) | ||||||
| Accounts payable, income taxes and other liabilities | 102,996 | 100,636 | 107,929 | |||||||||
| Pension obligation | (22,119 | ) | (64,971 | ) | (58,842 | ) | ||||||
| Other, net | 12,189 | 39,416 | 35,105 | |||||||||
| Net Cash Provided by Continuing Operating Activities | 1,236,048 | 1,104,173 | 1,220,749 | |||||||||
| Investing Activities | ||||||||||||
| Capital expenditures | (556,394 | ) | (457,067 | ) | (315,840 | ) | ||||||
| Capitalized software | (22,334 | ) | (22,454 | ) | (18,922 | ) | ||||||
| Change in short-term investments | (30,167 | ) | (18,633 | ) | (43,775 | ) | ||||||
| Purchases of long-term investments | (3,881 | ) | (9,672 | ) | (1,171 | ) | ||||||
| Acquisitions of businesses, net of cash acquired | (339,528 | ) | (231,464 | ) | | |||||||
| Proceeds from discontinued operations | 19,971 | | 62,051 | |||||||||
| Other, net | (85,922 | ) | (44,656 | ) | (62,566 | ) | ||||||
| Net Cash Used for Continuing Investing Activities | (1,018,255 | ) | (783,946 | ) | (380,223 | ) | ||||||
| Financing Activities | ||||||||||||
| Change in short-term debt | (121,102 | ) | 121,563 | 157,103 | ||||||||
| Payments of debt | (100,790 | ) | (828 | ) | (104,522 | ) | ||||||
| Repurchase of common stock | (450,124 | ) | (448,882 | ) | (549,999 | ) | ||||||
| Issuance of common stock | 130,679 | 147,796 | 123,494 | |||||||||
| Excess tax benefit from payments under share-based compensation plans | 55,118 | 50,609 | 40,594 | |||||||||
| Dividends paid | (239,810 | ) | (212,431 | ) | (182,236 | ) | ||||||
| Net Cash Used for Continuing Financing Activities | (726,029 | ) | (342,173 | ) | (515,566 | ) | ||||||
| Discontinued Operations: | ||||||||||||
| Net cash provided by (used for) operating activities | 4,388 | (27,773 | ) | (3,954 | ) | |||||||
| Net cash used for investing activities | | (2,580 | ) | (528 | ) | |||||||
| Net cash used for financing activities | | | (15 | ) | ||||||||
| Net Cash Provided by (Used for) Discontinued Operations | 4,388 | (30,353 | ) | (4,497 | ) | |||||||
| Effect of exchange rate changes on cash and equivalents | 15,041 | 9,698 | 3,049 | |||||||||
| Net (Decrease) Increase in Cash and Equivalents | (488,807 | ) | (42,601 | ) | 323,512 | |||||||
| Opening Cash and Equivalents | 1,000,289 | 1,042,890 | 719,378 | |||||||||
| Closing Cash and Equivalents | $ | 511,482 | $ | 1,000,289 | $ | 1,042,890 | ||||||
| See notes to consolidated financial statements | ||||||||||||
39
| Notes to Consolidated | Becton, Dickinson and Company | |
| Financial Statements |
Thousands of dollars, except per share amounts and numbers of shares
| 1 Summary of Significant Accounting Policies |
Principles of Consolidation
The consolidated financial statements
include the accounts of Becton, Dickinson and Company and its majority-owned
subsidiaries (the Company) after the elimination of inter-company
transactions. The Company has no material interests in variable interest entities
and none that require consolidation.
Cash Equivalents
Cash equivalents consist of all highly
liquid investments with a maturity of three months or less when purchased.
Short-Term Investments
Short-term investments consist of certificates
of deposit and repurchase agreements of government securities with maturities
of less than one year when purchased.
Inventories
Inventories are stated at the lower of
first-in, first-out cost or market.
Property, Plant and Equipment
Property, plant and equipment are stated
at cost, less accumulated depreciation and amortization. Depreciation and amortization
are principally provided on the straight-line basis over estimated useful lives,
which range from 20 to 45 years for buildings, four to 10 years for machinery
and equipment and two to 17 years for leasehold improvements. Depreciation
and amortization expense was $280,357, $262,956 and $242,063 in
fiscal 2007, 2006 and 2005, respectively.
Goodwill and Other Intangible
Assets
Goodwill is reviewed annually for impairment
in accordance with Statement of Financial Accounting Standards (SFAS)
No. 142, Goodwill and Other Intangible Assets. In reviewing goodwill
for impairment, potential impairment is identified by comparing the fair value
of a reporting unit, estimated using an income approach, with its carrying
value. Core and developed technology is amortized over periods ranging from
15 to 20 years, using the straight-line method. Both goodwill and core and
developed technology arise from acquisitions. Other
intangibles with finite useful lives, which include patents, are amortized over
periods principally ranging from two to 40 years, using the straight-line method.
These intangibles, including core and developed technology, are periodically
reviewed when impairment indicators are present to assess recoverability from
future operations using undiscounted cash flows in accordance with SFAS No. 144, Accounting
for the Impairment or Disposal of Long-Lived Assets. To the extent
carrying value exceeds the undiscounted cash flows, an impairment loss is
recognized in operating results based upon the excess of the carrying value
over fair value. Other intangibles also include certain trademarks that are
considered to have indefinite lives, as they are expected to generate cash
flows indefinitely, and are reviewed annually for impairment.
Capitalized Software
Capitalized software, including costs
for software developed or obtained for internal use is stated at cost, less
accumulated amortization. Amortization expense is principally provided on the
straight-line basis over estimated useful lives, which do not exceed 10 years.
Amortization expense was $66,386, $66,037 and $71,416 for 2007,
2006 and 2005, respectively.
Foreign Currency Translation
Generally, the net assets of foreign operations
are translated into U.S. dollars using current exchange rates. The U.S. dollar
results that arise from such translation, as well as exchange gains and losses
on intercompany balances of a long-term investment nature, are included in
the foreign currency translation adjustments in Accumulated other comprehensive
income (loss).
Revenue Recognition
Revenue from product sales is recognized
when title and risk of loss pass to the customer. For the sale of certain instruments
in the Biosciences segment, revenue is recognized upon completion of installation
at the customers site. Based upon the terms of other sales arrangements,
the Biosciences segment recognizes revenue in accordance with Emerging Issues
Task Force No. 00-21, Revenue Arrangements with Multiple Deliverables.
These sales arrangements have multiple deliverables and, as such, are divided
into separate units of accounting. Revenue and cost of products sold are recognized
at the completion of each deliverable based on the relative fair values of
items delivered.
40
| Notes to Consolidated Financial Statements Becton, Dickinson and Company | ||
The Companys domestic businesses sell products primarily to distributors who resell the products to end-user customers. Rebates are provided to distributors that sell to end-user customers at prices determined under a contract between the Company and the end-user customer. Provisions for rebates, as well as sales discounts and returns, are accounted for as a reduction of revenues when revenue is recognized.
Shipping and Handling Costs
Shipping and handling costs are included
in Selling and administrative expense. Shipping expense was $243,263, $219,788
and $216,239 in 2007, 2006 and 2005, respectively.
Derivative Financial Instruments
In accordance with SFAS No. 133, Accounting
for Derivative Instruments and Hedging Activities, as amended, all derivatives
are recorded in the balance sheet at fair value and changes in fair value are
recognized currently in earnings unless specific hedge accounting criteria
are met.
Derivative financial instruments are utilized by the Company in the management of its foreign currency and interest rate exposures. The Company hedges its foreign currency exposures by entering into offsetting forward exchange contracts and currency options when it deems appropriate. The Company utilizes interest rate swaps and forward rate agreements to manage its exposure to fluctuating interest rates. The Company does not use derivative financial instruments for trading or speculative purposes.
Any deferred gains or losses associated with derivative instruments, which on infrequent occasions may be terminated prior to maturity, are recognized in income in the period in which the underlying hedged transaction is recognized. In the event a designated hedged item is sold, extinguished or matures prior to the termination of the related derivative instrument, such instrument would be closed and the resultant gain or loss would be recognized in income.
Income Taxes
United States income taxes are not provided
on undistributed earnings of foreign subsidiaries where such undistributed
earnings are indefinitely reinvested outside the United States. Deferred taxes
are provided for earnings of foreign subsidiaries when those earnings are not
considered indefinitely reinvested. Income taxes are provided and tax credits
are recognized based on tax laws enacted at the dates of the financial statements.
The Company maintains valuation allowances where it is more likely than not that all or a portion of a deferred tax asset will not be realized. Changes in valuation allowances are included in our tax provision in the period of change. In determining whether a valuation allowance is warranted, management evaluates factors such as prior earnings history, expected future earnings, carryback and carryforward periods and tax strategies that could potentially enhance the likelihood of the realization of a deferred tax asset.
Earnings per Share
Basic earnings per share are computed
based on the weighted average number of common shares outstanding. Diluted
earnings per share reflect the potential dilution that could occur if securities
or other contracts to issue common stock were exercised or converted into common
stock.
Use of Estimates
The preparation of financial statements
in conformity with U.S. generally accepted accounting principles requires management
to make estimates and assumptions. These estimates or assumptions affect reported
assets, liabilities, revenues and expenses as reflected in the consolidated
financial statements. Actual results could differ from these estimates.
Share-Based Compensation
The Company accounts for all share-based
compensation under SFAS No. 123 (revised 2004)Share-Based Payment (SFAS
No. 123(R)). This statement requires the recognition of the fair value
of share-based compensation in net income. Compensation expense is recognized
on a straight-line basis over the requisite service period, which is generally
the vesting period.
41
| Notes to Consolidated Financial Statements Becton, Dickinson and Company | ||
| 2 Accounting Changes |
In September 2006, the Financial Accounting Standards Board (the FASB) issued SFAS No. 158 Employers Accounting for Defined Benefit Pension and Other Postretirement Plans, an amendment of FASB Statements No. 87, 88, 106 and 132(R) (SFAS No. 158). SFAS No. 158 requires the Company to recognize the overfunded or underfunded status of a defined benefit postretirement plan as an asset or liability in its Consolidated Balance Sheet and to recognize changes in the funded status in the year in which the changes occur through comprehensive income. SFAS No. 158 also requires the funded status of a plan to be measured as of the balance sheet date and provides for additional disclosure requirements. The Company adopted SFAS No. 158 on September 30, 2007. SFAS No. 158 will not change the measurement date of the Companys plans as the plans are measured at its fiscal year-end. See Note 5 regarding the Companys adoption of SFAS No. 158.
In March 2005, the FASB issued Interpretation No. 47 Accounting for Conditional Asset Retirement Obligations (FIN 47). FIN 47 clarifies that the term conditional asset retirement obligation as used in SFAS No. 143, Accounting for Asset Retirement Obligations refers to a legal obligation to perform an asset retirement activity in which the timing and/or method of settlement are conditional on a future event that may or may not be within the control of the Company. Accordingly, the Company is required to recognize a liability for the fair value of a conditional asset retirement obligation if the fair value can be reasonably estimated. The Company adopted this interpretation in the fourth quarter of 2006. The adoption of FIN 47 did not have a material impact on BDs consolidated financial statements.
Adoption of New Accounting
Standard
In July 2006, the FASB issued Interpretation
No. 48 Accounting for Uncertainty in Income Taxes (FIN 48).
FIN 48 prescribes guidance for recognition, measurement, and disclosure of
uncertain tax positions recognized in financial statements in accordance with
SFAS No. 109 Accounting for Income Taxes. This interpretation will
be applied to all tax positions upon its initial adoption. The Company adopted this interpretation
on October 1, 2007 and the cumulative effect of applying this interpretation
will be reported as an adjustment to the opening balance of retained earnings
for such fiscal year. Although, the Company is still evaluating the potential
impact of FIN 48, the decrease to opening retained earnings as of October
1, 2007, with a corresponding increase to the appropriate tax liability accounts,
is not expected to exceed $15 million.
| 3 Acquisitions and Divestitures |
TriPath
On December 20, 2006, the Company acquired
the outstanding shares (approximately 93.8%) of TriPath Imaging, Inc. (TriPath)
which it did not previously own. TriPath develops, manufactures, markets and
sells innovative solutions to improve the clinical management of cancer, including
detection, diagnosis, staging and treatment. The acquisition advances the Companys
position in cancer diagnostics. The acquisition was accounted for under the
purchase method of accounting and the results of operations of TriPath were
included in the Companys results as of the acquisition date. Pro forma
information was not provided as the acquisition did not have a material effect
on the Companys consolidated results. The purchase price was $361,883
in cash, including transaction costs and other consideration. The purchase
price was allocated based upon the fair values of the assets and liabilities
acquired. The allocation of the purchase price resulted in deferred tax assets
of $74,221 primarily consisting of net operating loss carry-forwards and
credits; core and developed technology of $135,097; deferred tax liabilities
of $52,662 primarily associated with other intangible assets; and other
net assets of $59,024 consisting primarily of cash and trade receivables.
Core and developed technology will be amortized on a straight-line basis over
its estimated useful life of
42
| Notes to Consolidated Financial Statements Becton, Dickinson and Company | ||
approximately 15 years. The excess of the purchase price over the fair value of the assets acquired of $31,464 was recorded as goodwill. The primary items that generated goodwill are the value of expanded product opportunities in oncology that are aligned with and complement ongoing research programs at the Company. The goodwill was allocated to the Diagnostics segment and is not deductible for tax purposes. As a result of settling a preacquisition legal contingency in the fourth quarter, the Company recorded an increase to other net assets and a decrease to goodwill of $7,167, which was reflected in the above allocation of the purchase price.
In connection with the acquisition, the Company also incurred a non-deductible charge of $114,739 for acquired in-process research and development. This charge, based on fair value, is associated with three projects: molecular Pap test, breast staging, and ovarian cancer detection. These projects had not yet reached technological feasibility and did not have alternative future use at the acquisition date. The portion of the charge allocated to each of these projects was $75,992, $18,764 and $19,983, respectively.
The molecular Pap test uses proprietary molecular biomarkers and reagents that are intended to allow for the primary screening of cervical cancer. The diagnostic assay is being developed to test slides prepared using TriPaths SurePath® liquid-based Pap test and to permit concurrent evaluation of morphologic features and measurement of the over-expression of molecular biomarkers that are associated with biopsy-proven moderate to severe cervical disease and cancer. Clinical trials have been initiated for this project.
The breast staging project uses proprietary molecular bio-markers and reagents that are intended to predict the risk of disease recurrence and to aid in treatment selection in patients with early stage breast cancer. The diagnostic assay is being developed for use with commercially available detection kits and staining platforms and will utilize TriPaths interactive histology imaging system to quantify biomarker over-expression in tissue samples collected at the time of initial diagnosis of breast cancer. Clinical trials have been initiated for this project.
The ovarian cancer detection project is intended to allow for serum-based screening and monitoring assays for ovarian cancer based upon the detection of multiple biomarkers using a proprietary panel of biomarkers and assay algorithms. In addition, multiplex testing platforms are being evaluated to allow for the simultaneous testing of multiple markers from a small volume of serum. The detection assays being developed will utilize certain technologies from the Biosciences segment. Clinical trials have not been initiated for this project.
The fair values of these projects were determined based upon the present value of projected cash flows utilizing an income approach reflecting the appropriate risk-adjusted discount rate based on the applicable technological and commercial risk of each project. These cash flows also took into account the income and expenses associated with the further development and commercialization of the underlying products. The range of discount rates assigned to the projects was 22 to 30 percent and gave consideration to the underlying risk relative to the developed technology, the overall commercial and technical risk, and the probabilities of success for each of the projects. The ongoing activity associated with each of these projects is not expected to be material to the Companys Research and development expense.
Other
On May 4, 2007, the Company acquired all
of the outstanding shares of Plasso Technology, Ltd. (Plasso),
a privately-held company that is developing the next generation of surface-critical
research tools utilizing functional coating technology for applications in
glycomics and cell culture, for $10,425 in cash including transaction costs.
In connection with the acquisition, the Company incurred a non-deductible charge
of $7,394 for acquired in-process research and development associated with
Plassos technology, for which, at the acquisition date, technological
feasibility had not been established and no alternative future use existed.
Because Plasso was a development stage company that had not commenced its planned
principal operations, the transaction was accounted for as an acquisition of
assets rather than as a business combination and, therefore, goodwill was not
recorded.
43
| Notes to Consolidated Financial Statements Becton, Dickinson and Company | ||
GeneOhm
On February 14, 2006, the Company acquired
all the outstanding stock of GeneOhm Sciences, Inc. (GeneOhm),
a company that develops molecular diagnostic testing for the rapid detection
of bacterial organisms, including those known to cause healthcare-associated
infections. The acquisition provides the Company with expanded entry into the
emerging field of healthcare-associated infections. The acquisition was accounted
for under the purchase method of accounting and the results of operations of
GeneOhm were included in the Companys results as of the acquisition date.
Pro forma information was not provided as the acquisition did not have a material
effect on the Companys consolidated results. The purchase price consisted
of an up-front cash payment of $232,542, including transaction costs, and
the purchase contract provides for additional contingent payments of up to $25,000,
based on future events occurring on or before December 31, 2007. The purchase
price was allocated based upon the fair values of the assets and liabilities
acquired. The allocation of the purchase price resulted in deferred tax assets
of $34,346 consisting of net operating loss carryforwards and credits;
other intangible assets, primarily core and developed technology, of $92,300;
deferred tax liabilities of $31,400 associated with other intangible assets,
and other net assets of $3,587. Core and developed technology will be amortized
on a straight-line basis over its estimated useful life of approximately 15
years. The excess of the purchase price over the fair value of the assets acquired
of $80,409 was recorded as goodwill. The primary items that generated goodwill
are the value of synergies in microbiology research and the expansion of product
offerings in molecular diagnostics. The goodwill was allocated to the Diagnostics
segment and is not deductible for tax purposes. In connection with the acquisition,
the Company also incurred a non-deductible charge of $53,300 for acquired
in-process research and development. This charge, based on fair value, is associated
with several products that have not reached technological feasibility and do
not have alternative future use at the acquisition date. The fair value of
each product was determined based upon the present value of projected cash
flows utilizing an income approach reflecting the appropriate risk-adjusted
discount rate based on the applicable technological and commercial risk of
each product. These cash flows took into account the income and expenses associated
with the further development and commercialization of the underlying products.
The ongoing activity associated with each of these products is not material
to the Companys research and development expense.
BGM
On September 28, 2006, the Company announced
a plan to exit the blood glucose monitoring (BGM) market. In accordance
with the plan, distribution of the BD Logic Blood
Glucose Monitor was immediately discontinued. BD will continue to distribute
test strips for its customers through December 2007. The decision to exit the
BGM market was made following an evaluation of the future outlook for the product
line. The Company recorded a pre-tax charge of $63,414 in 2006 in connection
with its decision to exit the BGM product line. This charge consisted of $5,352
related to estimated customer sales returns, $31,602 related to the write-off
of inventory and related purchase commitments, $14,052 related to long-lived
asset write-downs, and $12,408 related to severance and other exit costs.
During the fourth quarter of 2007, the Company reversed $8,781 of this
charge to reinstate certain long-lived assets to reflect the use of these assets.
At September
30, 2006, an accrual of $32,408, which primarily consisted of inventory-related
purchase commitments and severance, was reported in current liabilities. At
September 30, 2007, the accrual was substantially utilized, after reflecting
the reversal of $5,365 of these costs during 2007.
During the first quarter of 2007, the Company received an unsolicited offer for the purchase of the BGM product line. On December 11, 2006, the Company sold the product line for $19,971 and recognized a pre-tax gain on sale of $15,226. During the second quarter of 2007, the Company recognized adjustments, thereby increasing the gain on sale by $6,093. These adjustments constitute revisions to estimated sales return accruals, primarily related to obligations that ceased to exist in the second quarter pursuant to the sale terms. During 2007, adjustments of $3,226 were made to reduce other accruals related to obligations that remained with the Company upon divestiture of the product line. Additionally, the Company received a payment of $4,675, which represented the resolution of a contingency with a former supplier. Following the sale, the Companys prior period Consolidated Statements of Income and Cash Flows and related disclosures have been restated to separately present the results of the BGM product line as discontinued operations. The September 30, 2006 Consolidated Balance Sheet has not been restated.
44
Notes to Consolidated Financial Statements Becton, Dickinson and Company
Other
In August 2005, the Company completed
the sale of the Clontech unit of the Biosciences segment for $62,100 and
recognized a gain on sale of $13,336 ($28,533 after taxes). Clontechs
results of operations were reported as discontinued operations for all periods
presented in the accompanying Consolidated Statements of Income and Cash Flows.
Results of discontinued operations for the years ended September 30 were as follows:
| 2007 | 2006 | 2005 | (B) | ||||||
| Revenues | $ | 33,086 | $ | 96,811 | $ | 123,518 | |||
| Income (loss) from discontinued | |||||||||
| operations before income taxes | 49,108 | (95,653 | )(A) | (17,134 | ) | ||||
| Income tax (provision) benefit | (15,242 | ) | 32,823 | 26,877 | |||||
| Income (loss) from discontinued | |||||||||
| operations, net | $ | 33,866 | $ | (62,830 | )(A) | $ 9,743 |
| (A) | Includes post-closing charges of $4,708 ($3,311 after taxes) related to the divestiture of Clontech. |
| (B) | Includes revenues of $49,670 and income before taxes of $15,541 ($29,980 after taxes) related to the operations of Clontech. The effective tax rate benefit of 92.9% reflected the consummation of the sale of Clontech as a sale of stock, which was previously assumed to be an asset sale. The Company recognized a benefit from the write-off of deferred tax liabilities associated with basis adjustments. |
| 4 Other Intangible Assets |
Other intangible assets at September 30 consisted of:
| 2007 | 2006 | ||||||||||
| Gross | Gross | ||||||||||
| Carrying | Accumulated | Carrying | Accumulated | ||||||||
| Amount | Amortization | Amount | Amortization | ||||||||
| Amortized intangible | |||||||||||
| assets | |||||||||||
| Core and developed | |||||||||||
| technology | $ | 548,995 | $ | 174,216 | $ | 377,633 | $ | 132,822 | |||
| Patents, trademarks, | |||||||||||
| and other | 289,920 | 203,037 | 337,176 | 254,717 | |||||||
| $ | 838,915 | $ | 377,253 | $ | 714,809 | $ | 387,539 | ||||
| Unamortized intangible | |||||||||||
| assets | |||||||||||
| Trademarks | $ | 9,055 | $ | 9,042 | |||||||
Intangible amortization expense was $46,607, $34,843 and $29,529 in 2007, 2006 and 2005, respectively. The estimated aggregate amortization expense for the fiscal years ending September 30, 2008 to 2012 are as follows: 2008$49,100; 2009$46,900; 2010$45,200; 2011$43,700; 2012$40,600.
| 5 Benefit Plans |
The Company has defined benefit pension plans covering substantially all of its employees in the United States and certain foreign locations. The Company also provides certain postretirement healthcare and life insurance benefits to qualifying domestic retirees. Postretirement healthcare and life insurance benefit plans in foreign countries are not material. The measurement date used for the Companys employee benefit plans is September 30.
During 2007, the Company redesigned its U.S. pension plans to provide for a cash benefit formula by offering a one-time, irrevocable election to existing employees to change to this provision and mandating all new employees hired after April 1, 2007 to participate in the new formula. The Company also amended its other postretirement benefits plan to provide that new hires, as of April 1, 2007 or later, will no longer be eligible for company subsidized benefits. These amendments did not have a material impact on the net pension and postretirement cost of the Company.
45
Notes to Consolidated Financial Statements Becton, Dickinson and Company
Net pension and other postretirement cost for the years ended September 30 included the following components:
| Pension Plans | Other Postretirement Benefits | ||||||||||||||||||
| 2007 | 2006 | 2005 | 2007 | 2006 | 2005 | ||||||||||||||
| Service cost | $ | 69,869 | $ | 74,111 | $ | 61,836 | $ | 4,386 | $ | 4,164 | $ | 3,657 | |||||||
| Interest cost | 75,728 | 71,997 | 66,837 | 14,608 | 14,873 | 15,321 | |||||||||||||
| Expected return on plan assets | (88,527 | ) | (80,063 | ) | (59,372 | ) | | | | ||||||||||
| Amortization of prior service cost | 348 | 309 | 211 | (6,233 | ) | (6,233 | ) | (6,233 | ) | ||||||||||
| Amortization of loss | 17,507 | 27,932 | 22,951 | 5,795 | 7,127 | 6,164 | |||||||||||||
| Amortization of net obligation | (92 | ) | (70 | ) | 134 | | | | |||||||||||
| $ | 74,833 | $ | 94,216 | $ | 92,597 | $ | 18,556 | $ | 19,931 | $ | 18,909 | ||||||||
Net pension cost attributable to foreign plans included in the preceding table was $21,156, $18,639 and $16,772 in 2007, 2006 and 2005, respectively.
Effective September 30, 2007, the Company adopted the recognition and disclosure provisions of SFAS No. 158, which requires the Company to recognize on a prospective basis the funded status of its pension and other postretirement benefit plans in the Consolidated Balance Sheet with a corresponding adjustment to Accumulated other comprehensive income (loss). The Company also recognized the funded status of its postem-ployment benefit plans in connection with the adoption of SFAS No. 158. The minimum pension liability, previously included in Accumulated other comprehensive income (loss), and the related intangible asset were derecognized upon the adoption of SFAS No. 158.
The effects of applying SFAS No. 158 at September 30, 2007 were as follows:
| Before | SFAS | After | |||||||
| Application of | No. 158 | Application of | |||||||
| SFAS No. 158 | Adjustments | SFAS No. 158 | |||||||
| Prepaid expenses, deferred | |||||||||
| taxes and other | $ | 326,119 | $ | (186 | ) | $ | 325,933 | ||
| Other Intangibles, Net | 96,391 | (453 | ) | 95,938 | |||||
| Other | 502,428 | (35,836 | ) | 466,592 | |||||
| Salaries, wages and related items | (435,857 | ) | 3 | (435,854 | ) | ||||
| Long-Term Employee | |||||||||
| Benefit Obligations | (268,128 | ) | (176,746 | ) | (444,874 | ) | |||
| Deferred Income Taxes and Other | (91,535 | ) | 3,523 | (88,012 | ) | ||||
| Accumulated other comprehensive | |||||||||
| income | (211,523 | ) | 209,695 | (1,828 | ) |
46
Notes to Consolidated Financial Statements Becton, Dickinson and Company
The change in benefit obligation, change in fair value of plan assets, funded status and amounts recognized in the Consolidated Balance Sheets for these plans were as follows:
| Pension Plans | Other Postretirement Benefits | ||||||||||||
| 2007 | 2006 | 2007 | 2006 | ||||||||||
| Change in benefit obligation: | |||||||||||||
| Beginning obligation | $ | 1,384,667 | $ | 1,413,092 | $ |
255,726 | $ | 281,197 | |||||
| Service cost | 69,869 | 74,111 | 4,386 | 4,164 | |||||||||
| Interest cost | 75,728 | 71,997 | 14,608 | 14,873 | |||||||||
| Plan amendments | (16,586 | ) | 86 | | | ||||||||
| Benefits paid | (97,671 | ) | (75,207 | ) | (25,411 | ) | (22,734 | ) | |||||
| Actuarial gain | (63,519 | ) | (117,307 | ) | (11,818 | ) | (24,345 | ) | |||||
| Other, includes translation | 41,942 | 17,895 | 8,480 | 2,571 | |||||||||
| Benefit obligation at September 30 | $ | 1,394,430 | $ | 1,384,667 | $ |
245,971 | $ | 255,726 | |||||
| Change in fair value of plan assets: | |||||||||||||
| Beginning fair value | $ | 1,124,565 | $ | 933,920 | $ |
| | ||||||
| Actual return on plan assets | 138,446 | 91,569 | | | |||||||||
| Employer contribution | 96,952 | 160,340 | | | |||||||||
| Benefits paid | (97,671 | ) | (75,207 | ) | | | |||||||
| Other, includes translation | 33,877 | 13,943 | | | |||||||||
| Plan assets at September 30 | $ | 1,296,169 | $ | 1,124,565 | $ |
| | ||||||
| Funded status at September 30: | |||||||||||||
| Unfunded benefit obligation | $ | (98,261 | ) | $ | (260,102 | ) | $ |
(245,971 | ) | $ | (255,726 | ) | |
| Unrecognized net transition obligation | | (1,012 | ) | | | ||||||||
| Unrecognized prior service cost (credit) | | 6,193 | | (12,920 | ) | ||||||||
| Unrecognized net actuarial loss | | 356,968 | | 77,392 | |||||||||
| Net amount recognized | $ | (98,261 | ) | $ | 102,047 | $ |
(245,971 | ) | $ | (191,254 | ) | ||
| Amounts recognized in the Consolidated | |||||||||||||
| Balance Sheets at September 30: | |||||||||||||
| Other Intangibles, Net | $ | | $ | 2,345 | $ |
| | ||||||
| Other | 32,710 | 148,129 | | | |||||||||
| Salaries, wages and related items | (2,668 | ) | | (20,067 | ) | | |||||||
| Long-Term Employee Benefit Obligations | (128,303 | ) | (67,996 | ) | (225,904 | ) | (191,254 | ) | |||||
| Accumulated other comprehensive income (loss) before income taxes | | 19,569 | | | |||||||||
| Net amount recognized | $ | (98,261 | ) | $ | 102,047 | $ |
(245,971 | ) | $ | (191,254 | ) | ||
| Amounts recognized in Accumulated other comprehensive | |||||||||||||
| income (loss) before income taxes at September 30: | |||||||||||||
| Net transition obligation | $ | (1,156 | ) | $ |
| ||||||||
| Prior service credit | (10,086 | ) | (6,688 | ) | |||||||||
| Net actuarial loss | 238,144 | 62,194 | |||||||||||
| Net amount recognized | $ | 226,902 | $ |
55,506 | |||||||||
47
Notes to Consolidated Financial Statements Becton, Dickinson and Company
Foreign pension plan assets at fair value included in the preceding table were $359,291 and $299,047 at September 30, 2007 and 2006, respectively. The foreign pension plan projected benefit obligations were $430,265 and $382,584 at September 30, 2007 and 2006, respectively.
The projected benefit obligation, accumulated benefit obligation and fair value of plan assets for the pension plans with accumulated benefit obligations in excess of plan assets were $96,723, $76,398 and $14,685, respectively as of September 30, 2007, and $126,545, $100,473 and $41,576, respectively as of September 30, 2006.
The estimated net actuarial loss and prior service credit for pension benefits that will be amortized from Accumulated other comprehensive loss into net pension costs over the next fiscal year are expected to be $7,825 and $(1,117) million, respectively. The estimated net actuarial loss and prior service credit for other postretirement benefits that will be amortized from Accumulated other comprehensive income (loss) into net other postretirement costs over the next fiscal year are expected to be $3,949 and $(6,233) million, respectively.
The weighted average assumptions used in determining pension plan information were as follows:
| 2007 | 2006 | 2005 | |||||||
| Net Cost | |||||||||
| Discount rate: | |||||||||
| U.S. plans(A) | 5.95 | % | 5.50 | % | 6.00 | % | |||
| Foreign plans | 4.65 | 4.19 | 4.95 | ||||||
| Expected return on plan assets: | |||||||||
| U.S. plans | 8.00 | 8.00 | 8.00 | ||||||
| Foreign plans | 6.42 | 6.02 | 6.60 | ||||||
| Rate of compensation increase: | |||||||||
| U.S. plans(A) | 4.50 | 4.25 | 4.25 | ||||||
| Foreign plans | 3.08 | 2.92 | 2.98 | ||||||
| Benefit Obligation | |||||||||
| Discount rate: | |||||||||
| U.S. plans(A) | 6.35 | 5.95 | 5.50 | ||||||
| Foreign plans | 5.32 | 4.65 | 4.19 | ||||||
| Rate of compensation increase: | |||||||||
| U.S. plans(A) | 4.50 | 4.50 | 4.25 | ||||||
| Foreign plans | 3.45 | 3.08 | 2.92 | ||||||
| (A) Also used to determine other postretirement and postemployment benefit plan information. | |||||||||
At September 30, 2007 the assumed healthcare trend rates were 9% pre and post age 65, gradually decreasing to an ultimate rate of 5% beginning in 2012. At September 30, 2006 the corresponding assumed healthcare trend rates were 10% pre and post age 65, gradually decreasing to an ultimate rate of 5% beginning in 2012. A one percentage point increase in assumed healthcare cost trend rates in each year would increase the accumulated postretirement benefit obligation as of September 30, 2007 by $11,165 and the aggregate of the service cost and interest cost components of 2007 annual expense by $759. A one percentage point decrease in the assumed healthcare cost trend rates in each year would decrease the accumulated postretirement benefit obligation as of September 30, 2007 by $9,977 and the aggregate of the 2007 service cost and interest cost by $677.
Expected Funding
The Companys funding policy for its defined benefit
pension plans is to contribute amounts sufficient to meet legal funding
requirements, plus any additional amounts that may be appropriate considering
the funded status of the plans, tax consequences, the cash flow generated
by the Company and other factors. While the Company will not be required
to fund any of its pension plans in 2008, the Company made a discretionary
contribution to a certain foreign pension plan in October 2007 of $22,900.
Expected benefit payments are as follows:
| Other | ||||||
| Pension | Postretirement | |||||
| Plans | Benefits | |||||
| 2008 | $ | 81,738 | $ | 20,067 | ||
| 2009 | 70,735 | 20,378 | ||||
| 2010 | 75,948 | 20,798 | ||||
| 2011 | 81,612 | 20,907 | ||||
| 2012 | 89,059 | 20,757 | ||||
| 20132017 | 522,634 | 100,137 |
Expected receipts of the subsidy under the Medicare Prescription Drug Improvement and Modernization Act of 2003, which are not reflected in the expected other postretire-ment benefit payments included in the preceding table, are as follows: 2008, $2,059; 2009, $2,190; 2010, $2,280; 2011, $2,362; 2012, $2,417; 2013-2017, $12,174.
48
Notes to Consolidated Financial Statements Becton, Dickinson and Company
The Companys asset allocations for its defined benefit pension plans at September 30 were as follows
| 2007 | 2006 | |||||
| Equity securities | 64.5 | % | 64.4 | % | ||
| Debt securities | 33.1 | 33.0 | ||||
| Other | 2.4 | 2.6 | ||||
| 100.0 | % | 100.0 | % |
Investment Strategy
The Companys investment objective is to achieve
superior returns on plan assets, subject to a prudent level of portfolio
risk, for the purpose of enhancing the security of benefits for participants.
The Companys investments include a broad range of equity and fixed-income
securities. These investments are diversified in terms of domestic and
international equity securities, short-term and long-term securities, growth
and value styles, as well as small and large capitalization stocks. The
Companys target allocation percentages are as follows: equity securities
(58%69%); fixed-income securities (31%39%); and cash (0%3%).
Equity securities are held for their expected high return and excess return
over inflation. Fixed-income securities are held for diversification relative
to equities. The plans may also hold cash to meet liquidity requirements.
Due to short-term fluctuations in market conditions, allocation percentages
may temporarily deviate from these target allocation percentages before
a rebalancing occurs. Investment risks and returns are measured and monitored
on an on-going basis through annual liability measurements and quarterly
investment portfolio reviews to determine whether the asset allocation
targets continue to represent an appropriate balance of expected risk and
reward.
The expected rate of return on plan assets is based upon expectations of long-term average rates of return to be achieved by the underlying investment portfolios. In establishing this assumption, the Company considers historical and expected rates of return for the asset classes in which the plans assets are invested, as well as current economic and capital market conditions.
Postemployment Benefits
The Company utilizes a service-based approach in applying
SFAS No. 112, Employers Accounting for Postemployment Benefitsan
amendment of FASB Statements No. 5 and 43, for most of its postemployment
benefits. This approach recognizes that actuarial gains and losses may
result from experience that differs from baseline assumptions.
Postemployment benefit costs for the years ended September 30 included the following components:
| 2007 | 2006 | 2005 | ||||||
| Service cost | $ | 10,449 | $ | 10,148 | $ | 8,975 | ||
| Interest cost | 5,116 | 4,946 | 4,438 | |||||
| Amortization of prior service cost | 1,654 | 1,654 | 1,654 | |||||
| Amortization of loss | 6,895 | 8,548 | 7,613 | |||||
| $ | 24,114 | $ | 25,296 | $ | 22,680 |
The unfunded status of the postemployment benefit plans was $101,514 at September 30, 2007 and these plans are not funded. The amounts recognized in Accumulated other comprehensive income (loss) before income taxes for the net actuarial loss was $57,110 at September 30, 2007. The estimated net actuarial loss that will be amortized from the Accumulated other comprehensive income (loss) into postem-ployment benefit cost over the next fiscal year is $6,845.
Savings Incentive Plan
The Company has a voluntary defined contribution
plan (Savings Incentive Plan) covering eligible employees in the
United States. In connection with the redesign of the U.S. pension and postretirement
benefit plans, effective July 1, 2007, the Company amended its Savings Incentive
Plan increasing the amount of the Company matching contribution for eligible
employees to 75% of employees contributions, up to a maximum of 4.5%
of each employees eligible compensation. Prior to that date, the Company
matched 50% of employees contributions, up to a maximum of 3% of each
employees salary. The cost of the Savings Incentive Plan was $21,878
in 2007, $16,626 in 2006 and $6,905 in 2005. The Company guarantees
employees contributions to the fixed income fund of the Savings Incentive
Plan, which consists of diversified money market instruments. The amount guaranteed
was $144,772 at September 30, 2007.
49
Notes to Consolidated Financial Statements Becton, Dickinson and Company
| 6 Income Taxes |
The provision for income taxes from continuing operations for the years ended September 30 consisted of:
| 2007 | 2006 | 2005 | |||||||
| Current: | |||||||||
| Federal | $ | 307,072 | $ | 281,784 | $ | 130,657 | |||
| State and local, including Puerto Rico | 21,669 | 12,004 | 5,169 | ||||||
| Foreign | 134,526 | 125,289 | 125,414 | ||||||
| 463,267 | 419,077 | 261,240 | |||||||
| Deferred: | |||||||||
| Domestic | (94,306 | ) | (101,651 | ) | 76,540 | ||||
| Foreign | (21,183 | ) | (6,634 | ) | (12,771 | ) | |||
| (115,489 | ) | (108,285 | ) | 63,769 | |||||
| $ | 347,778 | $ | 310,792 | $ | 325,009 |
The components of Income From Continuing Operations Before Income Taxes for the years ended September 30 consisted of:
| 2007 | 2006 | 2005 | |||||||
| Domestic, including Puerto Rico | $ | 550,750 | $ | 466,655 | $ | 465,188 | |||
| Foreign | 653,195 | 659,247 | 572,341 | ||||||
| $ | 1,203,945 | $ | 1,125,902 | $ | 1,037,529 |
Deferred tax assets and liabilities are netted on the balance sheet by separate tax jurisdictions. At September 30, 2007 and 2006, net current deferred tax assets of $168,305 and $181,406, respectively, were included in Prepaid expenses, deferred taxes and other. Net non-current deferred tax assets of $168,251 and $32,582, respectively, were included in Other. Net current deferred tax liabilities of $6,136 and $2,184, respectively, were included in Current LiabilitiesIncome taxes. Net non-current deferred tax liabilities of $37,121 and $143,435, respectively, were included in Deferred Income Taxes and Other. Deferred taxes are not provided on undistributed earnings of foreign subsidiaries that are indefinitely reinvested. At September 30, 2007, the cumulative amount of such undistributed earnings indefinitely reinvested outside the United States was $1.6 billion. Determining the tax liability that would arise if these earnings were remitted is not practicable. Deferred taxes are provided for earnings outside the United States when those earnings are not considered indefinitely reinvested.
In October 2004, the American Jobs Creations Act of 2004 (the AJCA) was signed into law. The AJCA created a temporary incentive for U.S. multinationals to repatriate accumulated income earned outside the United States. As a result of the passage of the AJCA, the Company revisited its policy of indefinite reinvestment of foreign earnings and made a decision to repatriate approximately $1.3 billion in 2006 pursuant to its approved repatriation plan. The Company recorded a charge of $77,200 in 2005 attributable to the planned repatriation of these earnings. During 2006, the Company repatriated approximately $1.3 billion in accordance with its planned repatriation under the AJCA. The actual tax charge associated with this repatriation was $65,768.
Deferred income taxes at September 30 consisted of:
| 2007 | 2006 | ||||||||||
| Assets | Liabilities | Assets | Liabilities | ||||||||
| Compensation and benefits | $ | 301,118 | $ | | $ | 146,432 | $ | | |||
| Property and equipment | | 190,979 | | 144,365 | |||||||
| Loss and credit carryforwards | 193,981 | | 111,388 | | |||||||
| Other | 172,740 | 83,538 | 199,997 | 159,853 | |||||||
| 667,839 | 274,517 | 457,817 | 304,218 | ||||||||
| Valuation allowance | (100,023 | ) | | (85,230 | ) | | |||||
| $ | 567,816 | $ | 274,517 | $ | 372,587 | $ | 304,218 | ||||
Valuation allowances have been established for capital loss carryforwards, state deferred tax assets, net of federal tax, related to net operating losses and credits and other deferred tax assets for which the Company has determined it is more likely than not that these benefits will not be realized. At September 30, 2007, the Company had deferred state tax assets for net state operating losses and credit carryforwards of $49,641 for which a valuation allowance of $33,191 has been established due to the uncertainty of generating sufficient taxable income in the state jurisdictions to utilize the deferred tax assets before they principally expire between 2008 and 2014. In 2007, a previously established valuation allowance of approximately $19,700 related to state tax credit carryforwards was reversed and included in the state and local income tax line item in the following rate reconciliation table. The Company also has federal and state capital loss carryforward deferred tax assets of $51,428 for which a full valuation allowance has been established due to the uncertainty of recognizing the benefit from these losses before they principally expire in 2010.
50
Notes to Consolidated Financial Statements Becton, Dickinson and Company
A reconciliation of the federal statutory tax rate to the Companys effective tax rate was as follows:
| 2007 | 2006 | 2005 | |||||||
| Federal statutory tax rate | 35.0 | % | 35.0 | % | 35.0 | % | |||
| State and local income taxes, | |||||||||
| net of federal tax benefit | 0.2 | 0.6 | 0.7 | ||||||
| Effect of foreign and Puerto Rico | |||||||||
| earnings and foreign tax credits | (9.2 | ) | (7.4 | ) | (10.2 | ) | |||
| Effect of Research, Domestic Production | |||||||||
| Activities, Extraterritorial Income | |||||||||
| tax benefits | (0.5 | ) | (1.3 | ) | (2.0 | ) | |||
| Acquired in-process research | |||||||||
| and development | 3.6 | 1.8 | | ||||||
| Repatriation of foreign earnings | |||||||||
| under the AJCA | | (1.1 | ) | 7.7 | |||||
| Other, net | (0.2 | ) | | 0.1 | |||||
| 28.9 | % | 27.6 | % | 31.3 | % |
The approximate dollar and diluted earnings per share amounts of tax reductions related to tax holidays in various countries in which the Company does business were: 2007$80,300 and $0.32; 2006$70,000 and $0.27; and 2005$75,150 and $0.29. The tax holidays expire at various dates through 2023.
The Company made income
tax payments, net of refunds, of $345,049 in 2007, $398,808 in 2006
and $183,867 in 2005.
| 7 Supplemental Financial Information |
Other Income (Expense), Net
Other income (expense), net in 2007 was $944,
which primarily included income from license and other agreements of $6,128,
partially offset by net write downs of certain investments of $(5,538)
and foreign exchange losses (inclusive of hedging costs) of $(4,191).
Other income (expense), net in 2006 was $(8,762), which primarily included net write downs of certain investments of $(11,046) and foreign exchange losses (inclusive of hedging costs) of $(5,142), partially offset by income from license and other agreements of $4,281.
Other income (expense), net in 2005 was $(7,064), which primarily included foreign exchange losses (inclusive of hedging costs) of $(3,976) and net write downs of certain investments of $(3,519).
Trade Receivables, Net
Allowances for doubtful accounts and cash
discounts netted against trade receivables were $39,650 and $38,256
at September 30, 2007 and 2006, respectively.
Inventories
Inventories at September 30 consisted
of:
| 2007 | 2006 | |||||
| Materials | $ | 142,484 | $ | 121,598 | ||
| Work in process | 195,155 | 156,957 | ||||
| Finished products | 714,320 | 597,183 | ||||
| $ | 1,051,959 | $ | 875,738 |
Property, Plant and Equipment,
Net
Property, Plant and Equipment, Net at
September 30 consisted of:
| 2007 | 2006 | ||||
| Land | $ | 79,368 | $ | 68,882 | |
| Buildings | 1,597,356 | 1,361,614 | |||
| Machinery, equipment and fixtures | 3,596,781 | 3,239,397 | |||
| Leasehold improvements | 80,610 | 73,064 | |||
| 5,354,115 | 4,742,957 | ||||
| Less accumulated depreciation and amortization | 2,856,777 | 2,609,409 | |||
| $ | 2,497,338 | $ | 2,133,548 |
| 8 Debt |
Short-term debt at September 30 consisted of:
| 2007 | 2006 | |||||
| Loans payable: | ||||||
| Domestic | $ | 200,000 | $ | 200,000 | ||
| Foreign | 6,768 | 126,121 | ||||
| Current portion of long-term debt | 866 | 101,097 | ||||
| $ | 207,634 | $ | 427,218 |
Domestic loans payable consist of commercial paper. Foreign loans payable consist of short-term borrowings from financial institutions. The weighted average interest rates for Short-term debt were 5.2% and 4.6% at September 30, 2007 and 2006, respectively. During 2007, the Company amended its syndicated credit facility to increase the amount available from $900 million to $1 billion and extend the expiration date from August 2009 to December 2011. During 2008, the facility was again amended, extending its expiration date to December 2012. This credit facility provides backup support for the commercial paper program and can also be used for other general corporate purposes.
51
Notes to Consolidated Financial Statements Becton, Dickinson and Company
This credit facility includes a restrictive covenant that requires a minimum interest coverage ratio. There were no borrowings outstanding under the facility at September 30, 2007. In addition, the Company had short-term foreign lines of credit pursuant to informal arrangements of approximately $175,000 at September 30, 2007, of which $168,000 was unused.
Long-Term Debt at September 30 consisted of:
| 2007 | 2006 | |||||
| Domestic notes due through 2013 | ||||||
| (average year-end interest rate: | ||||||
| 4.3%2007; 4.2%2006) | $ | 9,801 | $ | 10,566 | ||
| 7.15% Notes due October 1, 2009 | 205,914 | 206,144 | ||||
| 4.55% Notes due April 15, 2013 | 198,734 | 198,537 | ||||
| 4.90% Notes due April 15, 2018 | 206,214 | 206,674 | ||||
| 7.00% Debentures due August 1, 2027 | 168,000 | 168,000 | ||||
| 6.70% Debentures due August 1, 2028 | 167,050 | 167,050 | ||||
| $ | 955,713 | $ | 956,971 |
Long-term debt balances as of September 30, 2007 and 2006 have been impacted by certain interest rate swaps that have been designated as fair value hedges, as discussed in Note 9.
The aggregate annual maturities of long-term debt during the fiscal years ending September 30, 2009 to 2012 are as follows: 2009$745; 2010$206,385; 2011$466; 2012$119.
The Company capitalizes interest costs as a component of the cost of construction in progress. A summary of interest costs for the years ended September 30 were as follows:
| 2007 | 2006 | 2005 | |||||||
| Charged to operations | $ | 46,420 | $ | 66,046 | $ | 55,673 | |||
| Capitalized | 27,528 | 19,955 | 14,770 | ||||||
| $ | 73,948 | $ | 86,001 | $ | 70,443 |
Interest paid, net of amounts capitalized, was $50,730 in 2007, $62,514 in 2006 and $68,527 in 2005.
| 9 Financial Instruments |
Foreign Exchange Derivatives
The Company uses foreign exchange forward
contracts and currency options to reduce the effect of fluctuating foreign
exchange rates on certain foreign currency denominated receivables and payables
and third party product sales. Gains and losses on the derivatives are intended
to offset gains and losses on the hedged transaction. The Companys foreign
currency risk exposure is in Europe, Asia Pacific, Canada, Japan, and Latin
America.
The Company hedges substantially all of its transactional foreign exchange exposures, primarily intercompany payables and receivables, through the use of forward contracts and currency options with maturities of less than 12 months. Gains or losses on these contracts are largely offset by gains and losses on the underlying hedged items. These foreign exchange contracts do not qualify for hedge accounting.
In addition, the Company enters into option and forward contracts to hedge certain forecasted sales that are denominated in foreign currencies. These contracts are designated as cash flow hedges and are effective as hedges of these revenues. These contracts are intended to reduce the risk that the Companys cash flows from certain third party transactions will be adversely affected by changes in foreign currency exchange rates. Changes in the effective portion of the fair value of these contracts are included in other comprehensive income until the hedged sales transactions are recognized in earnings. Once the hedged transaction occurs, the gain or loss on the contract is recognized from Accumulated other comprehensive income (loss) to revenues. The Company recorded hedge net gains, exclusive of hedging costs, of $6,911 and $8,242 and a net loss, exclusive of hedging costs, of $1,876 to revenues in 2007, 2006 and 2005, respectively. Revenues in 2007, 2006 and 2005 are net of hedging costs of $15,136, $12,508 and $17,286, respectively, related to the purchased option contracts. The Company records in Other income (expense), net, the premium or cost of the forward contracts, which is excluded from the assessment of hedge effectiveness. The net premium was $562 in 2006 and the net cost was $236 in 2005. All outstanding contracts that were designated as cash flow hedges as of September 30, 2007 will mature by September 30, 2008. At September 30, 2007 and 2006, Accumulated other comprehensive income (loss) included unrealized losses of $4,994 and $1,522, respectively, net of tax, relating to foreign exchange derivatives that have been designated as cash flow hedges.
52
Notes to Consolidated Financial Statements Becton, Dickinson and Company
Interest Rate Derivatives
The Companys policy is to manage
interest cost using a mix of fixed and floating rate debt. The Company has
entered into interest rate swaps in which it agrees to exchange, at specified
intervals, the difference between fixed and floating interest amounts calculated
by reference to an agreed-upon notional principal amount. These swaps are designated
as either fair value or cash flow hedges. For fair value hedges, changes in
the fair value of the interest rate swaps offset changes in the fair value
of the fixed rate debt due to changes in market interest rates. For cash flow
hedges, changes in the fair value of the interest rate swaps are offset by
amounts recorded in other comprehensive income (loss). There was no ineffective
portion to the hedges recognized in earnings during the period. If interest
rate derivatives designated as cash flow hedges mature or are terminated, then
the balance in other comprehensive income (loss) attributable to those derivatives
is reclassified into earnings over the remaining life of the hedged debt. The
amount that will be reclassified and recorded in Interest expense, net within
the next 12 months is $1,760.
At September 30, 2007 and 2006, Accumulated other comprehensive income (loss) included an unrealized loss of $11,397 and $12,273, respectively, net of tax, relating to interest rate derivatives that have been designated as cash flow hedges.
Fair Value of Financial Instruments
Cash equivalents, short-term investments
and short-term debt are carried at cost, which approximates fair value. Equity
securities, where a readily determinable market value exists, are classified
as available-for-sale securities. Available-for-sale securities are carried
at fair value, with unrecognized gains and losses reported in other comprehensive
income (loss), net of taxes. Losses on available-for-sale securities are recognized
when a loss is determined to be other than temporary or when realized.
The fair value of forward exchange contracts and currency options were estimated based on market prices, where available, or dealer quotes. The fair value of certain long-term debt is based on redemption value. The estimated fair values of the Companys financial instruments at September 30 were as follows:
| 2007 | 2006 | ||||||||||||
| Carrying | Fair | Carrying | Fair | ||||||||||
| Value | Value | Value | Value | ||||||||||
| Assets: | |||||||||||||
| Currency options(A) | $ | 3,982 | $ | 3,982 | $ | 12,471 | $ | 12,471 | |||||
| Forward exchange contracts(A) | 8,007 | 8,007 | 3,156 | 3,156 | |||||||||
| Interest rate swaps(A) | 5,914 | 5,914 | 6,144 | 6,144 | |||||||||
| Equity securities | 1,291 | 1,291 | 25,436 | (B) | 25,436 | (B) | |||||||
| Liabilities: | |||||||||||||
| Forward exchange contracts(C) | 8,968 | 8,968 | 2,878 | 2,878 | |||||||||
| Long-term debt | 955,713 | 949,490 | 956,971 | 976,404 | |||||||||
| (A) | Included in Prepaid expenses, deferred taxes and other. |
| (B) | Included in Other non-current assets and primarily represents equity securities in TriPath, acquired on December 20, 2006. |
| (C) | Included in Accrued Expenses. |
Concentration of Credit Risk
Substantially all of the Companys
trade receivables are due from public and private entities involved in the
healthcare industry. Due to the large size and diversity of the Companys
customer base, concentrations of credit risk with respect to trade receivables
are limited. The Company does not normally require collateral. The Company
is exposed to credit loss in the event of nonperformance by financial institutions
with which it conducts business. However, this loss is limited to the amounts,
if any, by which the obligations of the counterparty to the financial instrument
contract exceed the obligations of the Company. The Company also minimizes
exposure to credit risk by dealing with a diversified group of major financial
institutions.
53
Notes to Consolidated Financial Statements Becton, Dickinson and Company
| 10 Shareholders Equity |
Changes in certain components of shareholders equity were as follows:
| Common | ||||||||||||||||||
| Stock | Capital in | |||||||||||||||||
| Issued at | Excess of | Retained | Deferred | Treasury Stock | ||||||||||||||
| Par Value | Par Value | Earnings | Compensation | Shares | Amount | |||||||||||||
| Balance at September 30, 2004 | $ | 332,662 | $ | 414,515 | $ | 4,264,778 | $ | 10,222 | (83,327,295 | ) | $ | (1,816,756 | ) | |||||
| Net income | 722,263 | |||||||||||||||||
| Cash dividends: | ||||||||||||||||||
| Common ($.72 per share) | (181,189 | ) | ||||||||||||||||
| Common stock issued for: | ||||||||||||||||||
| Share-based compensation plans, net | 124,220 | 4,638,097 | 44,839 | |||||||||||||||
| Business acquisitions | 206 | 4,565 | 45 | |||||||||||||||
| Share-based compensation | 70,199 | |||||||||||||||||
| Common stock held in trusts, net | 58 | 40,472 | (58 | ) | ||||||||||||||
| Repurchase of common stock | (9,711,800 | ) | (549,999 | ) | ||||||||||||||
| Conversion of ESOP preferred stock | 6,706 | 3,378,028 | 24,436 | |||||||||||||||
| Balance at September 30, 2005 | $ | 332,662 | $ | 615,846 | $ | 4,805,852 | $ | 10,280 | (84,977,933 | ) | $ | (2,297,493 | ) | |||||
| Net income | 752,280 | |||||||||||||||||
| Cash dividends: | ||||||||||||||||||
| Common ($.86 per share) | (212,435 | ) | ||||||||||||||||
| Common stock issued for: | ||||||||||||||||||
| Share-based compensation plans, net | 148,342 | 5,066,384 | 49,057 | |||||||||||||||
| Business acquisitions | 734 | 15,864 | 156 | |||||||||||||||
| Share-based compensation | 108,613 | |||||||||||||||||
| Common stock held in trusts, net | 854 | (17,275 | ) | (854 | ) | |||||||||||||
| Repurchase of common stock | (7,281,100 | ) | (448,882 | ) | ||||||||||||||
| Balance at September 30, 2006 | $ | 332,662 | $ | 873,535 | $ | 5,345,697 | $ | 11,134 | (87,194,060 | ) | $ | (2,698,016 | ) | |||||
| Net income | 890,033 | |||||||||||||||||
| Cash dividends: | ||||||||||||||||||
| Common ($.98 per share) | (239,943 | ) | ||||||||||||||||
| Common stock issued for: | ||||||||||||||||||
| Share-based compensation plans, net | 143,420 | 4,380,724 | 43,213 | |||||||||||||||
| Business acquisitions | 707 | 10,812 | 105 | |||||||||||||||
| Share-based compensation | 107,706 | |||||||||||||||||
| Common stock held in trusts, net | 1,071 | (70,542 | ) | (1,071 | ) | |||||||||||||
| Repurchase of common stock | (5,952,000 | ) | (450,124 | ) | ||||||||||||||
| Balance at September 30, 2007 | $ | 332,662 | $ | 1,125,368 | $ | 5,995,787 | $ | 12,205 | (88,825,066 | ) | $ | (3,105,893 | ) | |||||
54
Notes to Consolidated Financial Statements Becton, Dickinson and Company
Common stock held in trusts represents rabbi trusts in connection with deferred compensation under the Companys employee salary and bonus deferral plan and directors deferral plan. In December 2004, the remaining unallocated shares of ESOP preferred stock were converted to BD common stock and were fully utilized by April 2005.
| 11 Accumulated Other Comprehensive Income (Loss) |
The components of Accumulated other comprehensive income (loss) were as follows:
| 2007 | 2006 | ||||||
| Foreign currency translation adjustments | $ | 237,394 | $ | (13,017 | ) | ||
| Minimum pension liability adjustment | | (12,059 | ) | ||||
| Benefit plans adjustment | (218,595 | ) | | ||||
| Unrealized (loss) gain on investments | (580 | ) | 10,063 | ||||
| Unrealized losses on cash flow hedges | (16,391 | ) | (13,795 | ) | |||
| $ | 1,828 | $ | (28,808 | ) |
The change in Accumulated other comprehensive income (loss) consists of other comprehensive income (loss) of $240,331, offset by the SFAS No. 158 adjustments of $209,695.
The income tax provision (benefit) recorded in fiscal years 2007 and 2006 for the unrealized gains on investments were $(6,524) and $743, respectively. The income tax benefit recorded in fiscal years 2007 and 2006 for cash flow hedges were $1,247 and $800, respectively. The income tax provision recorded in fiscal years 2007 and 2006 for the minimum pension liability adjustment were $2,050 and $47,259, respectively. Income taxes are generally not provided for translation adjustments.
The unrealized losses on cash flow hedges included in other comprehensive income (loss) for 2007 and 2006 are net of reclassification adjustments of $5,099 and $2,645, net of tax, respectively, for realized net hedge losses recorded to revenues. These amounts had been included in Accumulated other comprehensive income (loss) in prior periods. The tax benefits associated with these reclassification adjustments in 2007 and 2006 were $3,126 and $1,621, respectively.
| 12 Commitments and Contingencies |
Commitments
Rental expense for all operating leases
amounted to $68,100 in 2007, $63,400 in 2006, and $59,000 in 2005.
Future minimum rental commitments on noncancelable leases are as follows: 2008$45,600;
2009$34,200; 2010$25,200; 2011$18,300; 2012$14,100
and an aggregate of $16,200 thereafter.
As of September 30, 2007, the Company has certain future purchase commitments aggregating to approximately $365,000, which will be expended over the next several years.
Contingencies
The Company is named as a defendant in
five purported class action suits brought on behalf of direct purchasers of
the Companys products, such as distributors, alleging that the Company
violated federal antitrust laws, resulting in the charging of higher prices
for the Companys products to the plaintiff and other purported class
members. The cases filed are as follows: Louisiana
Wholesale Drug Company, Inc., et. al. vs. Becton Dickinson and Company (Civil
Action No. 05-1602, U.S. District Court, Newark, New Jersey), filed on March
25, 2005; SAJ Distributors, Inc. et. al.
vs. Becton Dickinson & Co. (Case 2:05-CV-04763-JD,
United States District Court, Eastern District of Pennsylvania), filed on September
6, 2005; Dik Drug Company, et. al. vs.
Becton, Dickinson and Company (Case No.
2:05-CV-04465, U.S. District Court, Newark, New Jersey), filed on September
12, 2005; American Sales Company, Inc.
et. al. vs. Becton, Dickinson & Co. (Case
No. 2:05-CV-05212-CRM, U.S. District Court, Eastern District of Pennsylvania),
filed on October 3, 2005; and Park Surgical
Co. Inc. et. al. vs. Becton, Dickinson and Company (Case
2:05-CV-05678-CMR, United States District Court, Eastern District of Pennsylvania),
filed on October 26, 2005.
The actions brought by Louisiana Wholesale Drug Company and Dik Drug Company in New Jersey have been consolidated under the caption In re Hypodermic Products Antitrust Litigation.
The Company is also named as a defendant in four purported class action suits brought on behalf of indirect purchasers of the Companys products, alleging that the Company violated federal antitrust laws, resulting in the charging of higher prices for the Companys products to the plaintiff and other purported class members. The cases filed are as follows: Jabos Pharmacy, Inc., et. al. v. Becton Dickinson & Company (Case No. 2:05-CV-00162, United States District Court, Greenville, Tennessee) filed on June 7, 2005; Drug Mart Tallman, Inc., et. al. v. Becton Dickinson and Company (Case No. 2:06-CV-00174, U.S. District Court, Newark, New Jersey), filed on January 17, 2006;
55
Notes to Consolidated Financial Statements Becton, Dickinson and Company
Medstar v. Becton Dickinson (Case No. 06-CV-03258-JLL (RJH), U.S. District Court, Newark, New Jersey), filed on May 18, 2006; and The Hebrew Home for the Aged at Riverdale v. Becton Dickinson and Company (Case No. 07-CV-2544, U.S. District Court, Southern District of New York), filed on March 28, 2007. A fifth purported class action on behalf of indirect purchasers (International Multiple Sclerosis Management Practice v. Becton Dickinson & Company (Case No. 2:07-cv-10602, U.S. District Court, Newark, New Jersey), filed on April 5, 2007) was voluntarily withdrawn by the plaintiff.
The plaintiffs in each of the antitrust class action lawsuits seek monetary damages. All of the antitrust class action lawsuits have been consolidated for pre-trial purposes in a Multi-District Litigation (MDL) in federal court in New Jersey.
On August 31, 2005, Daniels Sharpsmart filed suit against the Company, another manufacturer and three group purchasing organizations under the caption Daniels Sharpsmart, Inc. v. Tyco International, (US) Inc., et. al. (Civil Action No. 505CV169, United States District Court, Eastern District of Texas). The plaintiff alleged, among other things, that the Company and the other defendants conspired to exclude the plaintiff from the sharps-collection market by entering into long-term contracts in violation of federal and state antitrust laws, and sought monetary damages. On September 28, 2007, the Company and the plaintiff entered into an agreement to settle the matter on terms that are not material to the Company.
On June 6, 2006, UltiMed, Inc., a Minnesota company, filed suit against the Company in the United States District Court in Minneapolis, Minnesota (UltiMed, Inc. v. Becton, Dickinson and Company (06CV2266)). The plaintiff alleges, among other things, that the Company excluded the plaintiff from the market for home use insulin syringes by entering into anticompetitive contracts in violation of federal and state antitrust laws. The plaintiff seeks money damages and injunctive relief.
In June 2007, Retractable Technologies, Inc. (plaintiff) filed a complaint against the Company under the caption Retractable Technologies, Inc. vs. Becton Dickinson and Company (Civil Action No. 2:07-cv-250, United States District Court, Eastern District of Texas). Plaintiff alleges that the BD Integra syringes infringe patents licensed exclusively to the plaintiff. This patent claim was not covered by the release contained in the July 2004 settlement agreement between the Company and plaintiff to settle the lawsuit previously filed by plaintiff. In its complaint, plaintiff also alleges that the Company engaged in false advertising with respect to certain of the Companys safety-engineered products in violation of the Lanham Act; acted to exclude the plaintiff from various product markets and to maintain its market share through, among other things, exclusionary contracts in violation of state and Federal antitrust laws; and engaged in unfair competition. The non-patent claims purport to relate to actions allegedly taken by the Company following the date of the July 2004 settlement agreement referenced above. Plaintiff seeks treble damages, attorneys fees and injunctive relief.
The Company, along with another manufacturer and several medical product distributors, is named as a defendant in three product liability lawsuits relating to healthcare workers who allegedly sustained accidental needlesticks, but have not become infected with any disease. Generally, these actions allege that healthcare workers have sustained needlesticks using hollow-bore needle devices manufactured by the Company and, as a result, require medical testing, counseling and/or treatment. In some cases, these actions additionally allege that the healthcare workers have sustained mental anguish. Plaintiffs seek money damages in all of these actions. The Company had previously been named as a defendant in eight similar suits relating to healthcare workers who allegedly sustained accidental needle-sticks, each of which has either been dismissed with prejudice or voluntarily withdrawn. Regarding the three pending suits:
-
In Ohio, Grant vs. Becton Dickinson et. al. (Case No. 98CVB075616, Franklin County Court), on September 21, 2006, the Ohio Court of Appeals reversed the trial courts grant of class certification. The matter has been remanded to the trial court for a determination of whether the class can be redefined.
-
In Oklahoma and South Carolina, cases have been filed on behalf of an unspecified number of healthcare workers seeking class action certification under the laws of these states in state court in Oklahoma, under the caption Palmer vs. Becton Dickinson et. al. (Case No. CJ-98-685, Sequoyah County District Court), filed on October 27, 1998, and in state court in South Carolina, under the caption Bales vs. Becton Dickinson et. al. (Case No. 98-CP-40-4343, Richland County Court of Common Pleas), filed on November 25, 1998.
The Company continues to oppose class action certification in these cases, including pursuing all appropriate rights of appeal.
The Company, along with a number of other manufacturers, was named as a defendant in approximately 524 product liability lawsuits in various state and Federal courts related to natural rubber latex gloves which the Company ceased manufacturing in 1995. Cases pending in Federal court are being coordinated under the matter In re Latex Gloves Products Liability Litigation (MDL Docket No. 1148) in Philadelphia, and analogous procedures have been implemented in the state courts of California, Pennsylvania, New Jersey and New York. Generally, these actions allege that medical personnel have suffered allergic reactions ranging from skin irritation to anaphylaxis as a result of exposure to medical gloves containing natural rubber latex. Since the inception of this litigation, 467 of these cases have been closed with no liability to the Company, and 46 cases have been settled for an aggregate de minimis amount.
56
Notes to Consolidated Financial Statements Becton, Dickinson and Company
On August 8, 2005, the Company received a subpoena issued by the Attorney General of the State of Connecticut, which seeks documents and information relating to the Companys participation as a member of Healthcare Research & Development Institute, LLC (HRDI), a healthcare trade organization. The subpoena indicated that it was issued as part of an investigation into possible violations of the antitrust laws. On August 21, 2006, the Company received a subpoena issued by the Attorney General of the State of Illinois which sought documents and information relating to the Companys participation as a member of HRDI. The subpoena indicated that it was issued as part of an investigation into possible violations of the Illinois Consumer Fraud and Deceptive Business Practices Act, Charitable Trust Act, and Solicitation for Charity Act. An independent member of the Companys board of directors, Gary Mecklenburg, also served as a member and the non-executive chairman of HRDI until November 5, 2006. In January 2007, it was reported that HRDI entered into a settlement with the Attorneys General of Connecticut and Florida with respect to the investigation being conducted by the Connecticut Attorney General (the Company has not been contacted by the State of Florida). To the Companys knowledge, both the Connecticut and Illinois investigations are still ongoing. The Company believes that its participation in HRDI complied fully with the law and has responded to these subpoenas. The Company has not received any communication with respect to either investigation since completing its document production.
On May 28, 2004, Therasense, Inc. (Therasense) filed suit against the Company in the U.S. District Court for the Northern District of California (Case Number: C 04-02123 WDB) asserting that the Companys blood glucose monitoring products infringe certain Therasense patents. On August 10, 2004, in response to a motion filed by Therasense in the U.S. District Court for the District of Massachusetts, the court transferred to the court in California an action previously filed by the Company against Therasense requesting a declaratory judgment that the Companys products do not infringe the Therasense patents and that the Therasense patents are invalid.
As was previously reported, Becton Dickinson France, S.A., a subsidiary of the Company, was listed among approximately 2,200 other companies in an October 2005 report of the Independent Inquiry Committee (IIC) of the United Nations (UN) as having been involved in humanitarian contracts in which unauthorized payments were suspected of having been made to the Iraqi Government in connection with the UNs Oil-for-Food Programme (the Programme). The Company conducted an internal review and found no evidence that the Company or any employee or representative of the Company made, authorized, or approved improper payments to the Iraqi Government in connection with the Programme. The Company reported the results of its internal review to the Vendor Review Committee of the United Nations Procurement Service. In May 2007, the French Judicial Police conducted searches of the Companys offices in France with respect to the matters that were the subject of the 2005 IIC report. The Company was informed that it is one of a number of companies named in the IIC report that is being investigated by the French Judicial Police. The Company is cooperating fully with the investigation.
In July 2007, the Company received notice of a suit instituted in Saudi Arabia by El Seif Development (El Seif), a former distributor of the Company (Case No. 7516, Board of Grievances, Saudi Arabia). El Seif seeks monetary damages arising out of the termination of its distributor agreement and other contractual arrangements with the Company.
The Company has been served with a qui tam complaint filed by a private party against the Company in the United States District Court, Northern District of Texas, alleging violations of the Federal False Claims Act (FCA) and the Texas False Claims Act (the TFCA). Under the FCA, the United States Department of Justice, Civil Division has a certain period of time in which to decide whether to join the claim against the Company as an additional plaintiff; if not, the private plaintiff is free to pursue the claim on its own. A similar process is followed under the TFCA. To the Companys knowledge, no decision has yet been made by the Civil Division or the State of Texas whether to join this claim.
The Company believes that it has meritorious defenses to each of the above-mentioned suits pending against the Company and is engaged in a vigorous defense of each of these matters.
The Company is also involved both as a plaintiff and a defendant in other legal proceedings and claims that arise in the ordinary course of business.
The Company is a party to a number of Federal proceedings in the United States brought under the Comprehensive Environment Response, Compensation and Liability Act, also known as Superfund, and similar state laws. The affected sites are in varying stages of development. In some instances, the remedy has been completed, while in others, environmental studies are commencing. For all sites, there are other potentially responsible parties that may be jointly or severally liable to pay all cleanup costs.
Given the uncertain nature of litigation generally, the Company is not able in all cases to estimate the amount or range of loss that could result from an unfavorable outcome of the litigation to which the Company is a party. In accordance with U.S. generally accepted accounting principles, the Company establishes accruals to the extent probable future
57
Notes to Consolidated Financial Statements Becton, Dickinson and Company
losses are estimable (in the case of environmental matters, without considering possible third-party recoveries). In view of the uncertainties discussed above, the Company could incur charges in excess of any currently established accruals and, to the extent available, excess liability insurance. In the opinion of management, any such future charges, individually or in the aggregate, could have a material adverse effect on the Companys consolidated results of operations and consolidated cash flows.
| 13 Share-Based Compensation |
The Company grants share-based awards under the 2004 Employee and Director Equity-Based Compensation Plan (2004 Plan), which provides for long-term incentive compensation to employees and directors consisting of: stock appreciation rights (SARs), stock options, performance-based restricted stock units, time-vested restricted stock units and other stock awards. The Company believes such awards align the interest of its employees and directors with those of its shareholders. Prior to the adoption of the 2004 Plan, the Company had employee and director stock option plans, which were terminated with respect to future grants effective upon shareholder approval of the 2004 Plan in February 2004. In 2007, 2006 and 2005, the compensation expense for these plans charged to income was $107,706, $108,613 and $70,199, respectively, and the associated income tax benefit recognized was $37,179, $35,155 and $19,941, respectively.
Stock Appreciation Rights
Beginning with the annual share-based
grant in November 2005, the Company granted SARs and discontinued the issuance
of stock options. SARs represent the right to receive, upon exercise, shares
of common stock having a value equal to the difference between the market price
of common stock on the date of exercise and the exercise price on the date
of grant. SARs vest over a four-year period and have a ten-year term, similar
to the previously granted stock options. The fair value was estimated on the
date of grant using a lattice-based binomial option valuation model that uses
the following weighted-average assumptions in 2007 and 2006: risk-free interest
rate of 4.56% and 4.48%, respectively; expected volatility of 28% for both
years; expected dividend yield of 1.37% and 1.46%, respectively, and expected
life of 6.5 years for both years. Expected volatility is based upon historical
volatility for the Companys common stock and other factors.
The expected term of SARs granted is derived from the output of the model, using assumed exercise rates based on historical exercise and termination patterns, and represents the period of time that options granted are expected to be outstanding. The risk-free interest rate used is based upon the published U.S. Treasury yield curve in effect at the time of grant for instruments with a similar life. The dividend yield is based upon the most recently declared quarterly dividend as of the grant date. The weighted average grant date fair value of SARs granted during 2007 and 2006 was $22.66 and $18.43, respectively. The total intrinsic value of SARs exercised during 2007 was $321. The Company issued 3,192 shares during 2007 to satisfy the SARs exercised.
A summary of SARs outstanding as of September 30, 2007, and changes during the year then ended is as follows:
| Weighted | ||||||||||
| Weighted | Average | |||||||||
| Average | Remaining | Aggregate | ||||||||
| Grant Date | Contractual | Intrinsic | ||||||||
| SARs | Fair Value | Term (Years) | Value | |||||||
| Balance at October 1 | 1,684,541 | $ | 59.16 | |||||||
| Granted | 1,570,336 | 71.75 | ||||||||
| Exercised | (18,882 | ) | 59.16 | |||||||
| Forfeited, canceled | ||||||||||
| or expired | (71,266 | ) | 65.62 | |||||||
| Balance at | ||||||||||
| September 30 | 3,164,729 | $ | 65.26 | 8.63 | $ | 53,137 | ||||
| Vested and expected to | ||||||||||
| vest at September 30 | 2,896,169 | $ | 65.17 | 8.62 | $ | 48,885 | ||||
| Exercisable at | ||||||||||
| September 30 | 479,130 | $ | 59.90 | 8.20 | $ | 10,613 |
Stock Options
All stock option grants are for a ten-year
term. Stock options issued after November 2001 vest over a four-year period.
Stock options issued prior to November 2001 vested over a three-year period.
Stock options granted in 2005 were valued based on the grant date fair value
of those awards, using a lattice-based binomial option valuation model that
used the following weighted-average assumptions: risk-free interest rate of
3.93%; expected volatility of 29%; expected dividend yield of 1.28% and expected
life of 6.5 years.
The weighted average grant date fair value of stock options granted during 2005 was $17.16.
58
Notes to Consolidated Financial Statements Becton, Dickinson and Company
A summary of stock options outstanding as of September 30, 2007, and changes during the year then ended is as follows:
| Weighted | ||||||||||
| Weighted | Average | |||||||||
| Average | Remaining | Aggregate | ||||||||
| Stock | Exercise | Contractual | Intrinsic | |||||||
| Options | Price | Term (Years) | Value | |||||||
| Balance at October 1 | 18,253,990 | $ | 34.90 | |||||||
| Granted | | | ||||||||
| Exercised | (4,213,955 | ) | 31.83 | |||||||
| Forfeited, canceled | ||||||||||
| or expired | (42,288 | ) | 39.70 | |||||||
| Balance at | ||||||||||
| September 30 | 13,997,747 | $ | 35.81 | 4.75 | $ | 647,241 | ||||
| Vested and expected to | ||||||||||
| vest at September 30 | 13,826,293 | $ | 35.68 | 4.73 | $ | 641,058 | ||||
| Exercisable at | ||||||||||
| September 30 | 12,283,204 | $ | 34.39 | 4.49 | $ | 585,412 |
Cash received from the exercising of stock options in 2007, 2006 and 2005 was $134,133, $147,831 and $123,613, respectively. The actual tax benefit realized for tax deductions from stock option exercises totaled $59,491, $48,751 and $44,958, respectively. The total intrinsic value of stock options exercised during the years 2007, 2006 and 2005 was $187,537, $168,752 and $134,342, respectively.
Performance-Based Restricted
Stock Units
Performance-based restricted stock units
cliff vest three years after the date of grant. These units are tied to the
Companys performance against pre-established targets, including its average
growth rate of consolidated revenues and average return on invested capital,
over a three-year performance period. Under the Companys long-term incentive
program, the actual payout under these awards may vary from zero to 250% of
an employees target payout, based on the Companys actual performance
over the three-year performance period. The fair value is based on the market
price of the Companys stock on the date of grant. Compensation cost initially
recognized assumes that the target payout level will be achieved and is adjusted
for subsequent changes in the expected outcome of performance-related conditions.
A summary of performance-based restricted stock units outstanding as of September 30, 2007, and changes during the year then ended is as follows:
| Weighted | |||||
| Average | |||||
| Stock | Grant Date | ||||
| Units | Fair Value | ||||
| Balance at October 1 | 3,013,113 | $ | 54.62 | ||
| Granted | 1,210,648 | 71.72 | |||
| Vested | (120,923 | ) | 39.71 | ||
| Forfeited or canceled | (218,883 | ) | 57.84 | ||
| Balance at September 30(A) | 3,883,955 | $ | 60.23 | ||
| Expected to vest at September 30(B) | 1,954,149 | $ | 58.11 |
| (A) | Based on 250% of the target payout. |
| (B) | Net of expected forfeited units and units in excess of the expected performance payout of 241,858 and 1,687,948, respectively. |
The weighted average grant date fair value of performance-based restricted stock units granted during the years 2006 and 2005 was $59.16 and $54.41, respectively. At September 30, 2007, the weighted average remaining contractual term of performance-based restricted stock units is 1.10 years.
Time-Vested Restricted Stock
Units
Time-vested restricted stock units generally
cliff vest three years after the date of grant, except for certain key executives
of the Company, including the executive officers, for which such units generally
vest one year following the employees retirement. The related share-based
compensation expense is recorded over the requisite service period, which is
the vesting period or in the case of certain key executives is based on retirement
eligibility. The fair value of all time-vested restricted stock units is based
on the market value of the Companys stock on the date of grant.
A summary of time-vested restricted stock units outstanding as of September 30, 2007, and changes during the year then ended is as follows:
| Weighted | |||||
| Average | |||||
| Stock | Grant Date | ||||
| Units | Fair Value | ||||
| Balance at October 1 | 1,166,718 | $ | 55.95 | ||
| Granted | 540,853 | 72.20 | |||
| Vested | (42,944 | ) | 55.83 | ||
| Forfeited or canceled | (46,545 | ) | 72.03 | ||
| Balance at September 30 | 1,618,082 | $ | 61.11 | ||
| Expected to vest at September 30 | 1,456,274 | $ | 61.11 |
59
Notes to Consolidated Financial Statements Becton, Dickinson and Company
The weighted average grant date fair value of time-vested restricted stock units granted during the years 2006 and 2005 was $59.62 and $54.48, respectively. At September 30, 2007, the weighted average remaining contractual term of the time-vested restricted stock units is 2.03 years.
The amount of unrecognized compensation expense for all non-vested share-based awards as of September 30, 2007, is approximately $108.4 million, which is expected to be recognized over a weighted-average remaining life of approximately 1.95 years. At September 30, 2007, 6,420,643 shares were authorized for future grants under the 2004 Plan.
The Company has a policy of satisfying share-based payments through either open market purchases or shares held in treasury. At September 30, 2007, the Company has sufficient shares held in treasury to satisfy these payments in 2008.
Other Stock Plans
The Company has a Stock Award Plan, which
allows for grants of common shares to certain key employees. Distribution of
25% or more of each award is deferred until after retirement or involuntary
termination, upon which the deferred portion of the award is distributable
in five equal annual installments. The balance of the award is distributable
over five years from the grant date, subject to certain conditions. In February
2004, this plan was terminated with respect to future grants upon the adoption
of the 2004 Plan. At September 30, 2007 and 2006, awards for 214,206 and 270,762
shares, respectively, were outstanding.
The Company has a Restricted Stock Plan for Non-Employee Directors which reserves for issuance of 300,000 shares of the Companys common stock. No restricted shares were issued in 2007.
The Company has a Directors Deferral Plan, which provides a means to defer director compensation, from time to time, on a deferred stock or cash basis. As of September 30, 2007, 117,044 shares were held in trust, of which 3,466 shares represented Directors compensation in 2007, in accordance with the provisions of the plan. Under this plan, which is unfunded, directors have an unsecured contractual commitment from the Company.
The Company also has a Deferred Compensation Plan that allows certain highly-compensated employees, including executive officers, to defer salary, annual incentive awards and certain equity-based compensation. As of September 30, 2007, 265,846 shares were issuable under this plan.
| 14 Earnings per Share |
The weighted average common shares used in the computations of basic and diluted earnings per share (shares in thousands) for the years ended September 30 were as follows:
| 2007 | 2006 | 2005 | ||||
| Average common shares outstanding | 244,929 | 247,067 | 251,429 | |||
| Dilutive share equivalents from | ||||||
| share-based plans | 9,881 | 9,487 | 9,283 | |||
| Average common and common equivalent | ||||||
| shares outstandingassuming dilution | 254,810 | 256,554 | 260,712 |
| 15 Segment Data |
The Companys organizational structure is based upon its three principal business segments: BD Medical (Medical), BD Diagnostics (Diagnostics) and BD Biosciences (Biosciences).
The principal product lines in the Medical segment include needles, syringes and intravenous catheters for medication delivery; prefilled IV flush syringes; syringes and pen needles for the self-injection of insulin and other drugs used in the treatment of diabetes; prefillable drug delivery devices provided to pharmaceutical companies and sold to end-users as drug/device combinations; surgical blades/scalpels and regional anesthesia needles and trays; critical care monitoring devices; ophthalmic surgical instruments; sharps disposal containers; and home healthcare products. The principal products and services in the Diagnostics segment include integrated systems for specimen collection; an extensive line of safety-engineered specimen blood collection products and systems; plated media; automated blood culturing systems; molecular testing systems for sexually transmitted diseases and healthcare-associated infections; microorganism identification and drug susceptibility systems; liquid-based cytology systems for cervical cancer screening; and rapid diagnostic assays. The principal product lines in the Biosciences segment include fluorescence activated cell sorters and analyzers; cell imaging systems; monoclonal antibodies and kits for performing cell analysis; reagent systems for life sciences research; tools to aid in drug discovery and growth of tissue and cells; cell culture media supplements for biopharmaceutical manufacturing; and diagnostic assays.
The Company evaluates performance of its business segments based upon operating income. Segment operating income represents revenues reduced by product costs and operating expenses.
60
Notes to Consolidated Financial Statements Becton, Dickinson and Company
Distribution of products is primarily through independent sales representatives, independent distribution channels, and directly to other end-users. Sales to a distributor that supplies products from the Medical and Diagnostics segments accounted for approximately 9% of revenues in 2007, and 11% of revenues in 2006 and 2005, respectively. No other customer accounted for 10% or more of revenues in any of the three years presented.
| Revenues(A) | 2007 | 2006 | 2005 | ||||||
| Medical | $ | 3,420,670 | $ | 3,106,646 | $ | 2,884,240 | |||
| Diagnostics | 1,905,105 | 1,715,090 | 1,632,260 | ||||||
| Biosciences | 1,033,933 | 916,281 | 824,333 | ||||||
| $ | 6,359,708 | $ | 5,738,017 | $ | 5,340,833 | ||||
| Segment Operating Income | |||||||||
| Medical | $ | 971,990 | $ | 864,180 | $ | 747,777 | |||
| Diagnostics | 342,778 | (B) | 390,355 | (B) | 403,420 | ||||
| Biosciences | 258,806 | (B) | 221,925 | 185,827 | |||||
| Total Segment Operating Income | 1,573,574 | 1,476,460 | 1,337,024 | ||||||
| Unallocated Expenses(C) | (369,629 | ) | (350,558 | ) | (299,495 | ) | |||
| Income From Continuing Operations | |||||||||
| Before Income Taxes | $ | 1,203,945 | $ | 1,125,902 | $ | 1,037,529 | |||
| Segment Assets | |||||||||
| Medical | $ | 3,289,490 | $ | 2,835,613 | $ | 2,656,320 | |||
| Diagnostics | 1,843,654 | 1,485,959 | 1,245,769 | ||||||
| Biosciences | 817,000 | 727,634 | 678,286 | ||||||
| Total Segment Assets | 5,950,144 | 5,049,206 | 4,580,375 | ||||||
| Corporate and All Other(D) | 1,379,221 | 1,775,319 | 1,552,418 | ||||||
| $ | 7,329,365 | $ | 6,824,525 | $ | 6,132,793 | ||||
| Capital Expenditures | |||||||||
| Medical | $ | 352,696 | $ | 268,669 | $ | 182,737 | |||
| Diagnostics | 113,691 | 104,815 | 99,742 | ||||||
| Biosciences | 73,502 | 38,952 | 22,218 | ||||||
| Corporate and All Other | 16,505 | 44,631 | 11,143 | ||||||
| $ | 556,394 | $ | 457,067 | $ | 315,840 | ||||
| Depreciation and Amortization | |||||||||
| Medical | $ | 223,430 | $ | 210,044 | $ | 197,998 | |||
| Diagnostics | 138,936 | 116,072 | 102,882 | ||||||
| Biosciences | 68,889 | 63,383 | 64,599 | ||||||
| Corporate and All Other | 10,086 | 12,833 | 17,190 | ||||||
| $ | 441,341 | $ | 402,332 | $ | 382,669 |
| (A) | Intersegment revenues are not material. |
| (B) | Includes the acquired in-process research and development charges in 2007 related to the TriPath and Plasso acquisitions, and in 2006 related to the GeneOhm acquisition, as discussed in Note 3. |
| (C) | Includes primarily share-based compensation expense; interest, net; foreign exchange; and corporate expenses. |
| (D) | Includes cash and investments and corporate assets. |
| Revenues by Organizational Units | 2007 | 2006 | 2005 | |||||
| BD Medical | ||||||||
| Medical Surgical Systems | $ | 1,864,080 | $ | 1,748,743 | $ | 1,661,150 | ||
| Diabetes Care | 695,981 | 656,533 | 600,172 | |||||
| Pharmaceutical Systems | 791,900 | 639,694 | 563,271 | |||||
| Ophthalmic Systems | 68,709 | 61,676 | 59,647 | |||||
| $ | 3,420,670 | $ | 3,106,646 | $ | 2,884,240 | |||
| BD Diagnostics | ||||||||
| Preanalytical Systems | $ | 1,006,692 | $ | 927,759 | $ | 854,831 | ||
| Diagnostic Systems | 898,413 | 787,331 | 777,429 | |||||
| $ | 1,905,105 | $ | 1,715,090 | $ | 1,632,260 | |||
| BD Biosciences | ||||||||
| Immunocytometry Systems | $ | 588,401 | $ | 502,847 | $ | 452,383 | ||
| Discovery Labware | 277,902 | 256,085 | 231,365 | |||||
| Pharmingen | 167,630 | 157,349 | 140,585 | |||||
| $ | 1,033,933 | $ | 916,281 | $ | 824,333 | |||
| $ | 6,359,708 | $ | 5,738,017 | $ | 5,340,833 |
Geographic Information
The countries in which the Company has
local revenue-generating operations have been combined into the following geographic
areas: the United States (including Puerto Rico), Europe, and Other, which
is composed of Canada, Latin America, Japan and Asia Pacific.
Revenues to unaffiliated customers are based upon the source of the product shipment. Long-lived assets, which include net property, plant and equipment, are based upon physical location.
| 2007 | 2006 | 2005 | ||||||
| Revenues | ||||||||
| United States | $ | 3,033,005 | $ | 2,739,344 | $ | 2,521,672 | ||
| Europe | 2,047,388 | 1,762,782 | 1,670,963 | |||||
| Other | 1,279,315 | 1,235,891 | 1,148,198 | |||||
| $ | 6,359,708 | $ | 5,738,017 | $ | 5,340,833 | |||
| Long-Lived Assets | ||||||||
| United States | $ | 2,172,327 | $ | 1,934,994 | $ | 1,687,808 | ||
| Europe | 1,106,284 | 893,495 | 823,694 | |||||
| Other | 646,188 | 540,925 | 424,165 | |||||
| Corporate | 274,000 | 269,858 | 221,812 | |||||
| $ | 4,198,799 | $ | 3,639,272 | $ | 3,157,479 |
61
Becton, Dickinson and Company
| Quarterly Data (unaudited) | |||||||||||||||
| Thousands of dollars, except per share amounts | |||||||||||||||
| 2007 | |||||||||||||||
| 1st | 2nd | 3rd | 4th | Year | |||||||||||
| Revenues | $ | 1,501,526 | $ | 1,575,922 | $ | 1,631,159 | $ | 1,651,101 | $ | 6,359,708 | |||||
| Gross Profit | 792,593 | 811,382 | 840,088 | 843,724 | 3,287,787 | ||||||||||
| Income from Continuing Operations | 131,051 | (B) | 235,539 | 240,469 | (B) | 249,108 | 856,167 | (B) | |||||||
| Earnings per Share: | |||||||||||||||
| Income from Continuing Operations | .53 | .96 | .98 | 1.02 | 3.50 | ||||||||||
| Income from Discontinued Operations | .05 | .03 | .02 | .04 | .14 | ||||||||||
| Basic Earnings per Share | .58 | .99 | 1.00 | 1.07 | 3.63 | ||||||||||
| Income from Continuing Operations | .51 | .92 | .95 | .98 | 3.36 | ||||||||||
| Income from Discontinued Operations | .05 | .03 | .02 | .04 | .13 | ||||||||||
| Diluted Earnings per Share(A) | .56 | .95 | .96 | 1.03 | 3.49 | ||||||||||
| 2006 | |||||||||||||||
| 1st | 2nd | 3rd | 4th | Year | |||||||||||
| Revenues | $ | 1,393,845 | $ | 1,424,209 | $ | 1,457,347 | $ | 1,462,616 | $ | 5,738,017 | |||||
| Gross Profit | 727,899 | 725,443 | 737,832 | 753,578 | 2,944,752 | ||||||||||
| Income from Continuing Operations | 223,702 | 163,458 | (C) | 211,070 | 216,880 | 815,110 | (C) | ||||||||
| Earnings per Share: | |||||||||||||||
| Income from Continuing Operations | .90 | .66 | .86 | .88 | 3.30 | ||||||||||
| Loss from Discontinued Operations | (.02 | ) | (.04 | ) | (.02 | ) | (.17 | )(D) | (.25 | )(D) | |||||
| Basic Earnings per Share(A) | .88 | .62 | .84 | .71 | 3.04 | ||||||||||
| Income from Continuing Operations | .87 | .63 | .83 | .85 | 3.18 | ||||||||||
| Loss from Discontinued Operations | (.02 | ) | (.04 | ) | (.02 | ) | (.17 | )(D) | (.24 | )(D) | |||||
| Diluted Earnings per Share(A) | .85 | .60 | .81 | .68 | 2.93 |
| (A) | Total per share amounts may not add due to rounding. |
| (B) | Includes the acquired in-process research and development charges in the first and third quarters related to the TriPath and Plasso acquisitions, respectively, as discussed in Note 3. |
| (C) | Includes the acquired in-process research and development charge related to the GeneOhm acquisition, as discussed in Note 3. |
| (D) | Includes the impact of the BGM exit costs, as discussed in Note 3. |
62
| Corporate Information | Becton, Dickinson and Company |
Annual Meeting
1:00 p.m.
Tuesday, January 29, 2008
Hilton Short Hills
41 John F. Kennedy Parkway
Short Hills, NJ 07078
This annual report is not a solicitation of proxies.
Direct Stock Purchase Plan
The Direct Stock Purchase Plan established
through Computershare Trust Company, N.A., enhances the services provided to
existing shareholders and facilitates initial investments in BD shares. Plan
documentation and additional information may be obtained by calling Computershare
Trust Company, N.A., at 1-877-498-8861, or by accessing the Buy Shares feature
located within the Investor Centre of Computershares website at www.computershare.com.
NYSE Symbol
BDX
On February 22, 2007, Edward J. Ludwig, Chairman, President and Chief Executive Officer, submitted to the NYSE the Written Affirmation required by the rules of the NYSE certifying that he was not aware of any violations by BD of NYSE Corporate Governance listing standards.
The certifications of Mr. Ludwig and John R. Considine, Senior Executive Vice President and Chief Financial Officer, made pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 regarding the quality of BDs public disclosure, have been filed as exhibits to the Companys 2007 Annual Report on Form 10-K.
Transfer Agent and Registrar
Computershare Trust Company, N.A.
250 Royall Street
Canton, MA 02021
Phone: 1-877-498-8861
International: 1-781-575-2726
Internet: www.computershare.com
| Common Stock Prices and Dividends (per common share) | ||||||||
| By Quarter | 2007 | |||||||
| High | Low | Dividends | ||||||
| First | $ | 73.79 | $ | 68.81 | $ | 0.245 | ||
| Second | 78.14 | 69.85 | 0.245 | |||||
| Third | 80.87 | 73.65 | 0.245 | |||||
| Fourth | 82.61 | 74.24 | 0.245 | |||||
| By Quarter | 2006 | |||||||
| High | Low | Dividends | ||||||
| First | $ | 60.72 | $ | 50.07 | $ | 0.215 | ||
| Second | 65.76 | 58.97 | 0.215 | |||||
| Third | 65.28 | 58.31 | 0.215 | |||||
| Fourth | 70.67 | 58.84 | 0.215 |
Shareholder Information
At November 14, 2007, BD had approximately 8,862 shareholders of record. BDs Statement of Corporate Governance Principles, BDs Business Conduct and Compliance Guide, the charters of BDs Committees of the Board of Directors, BDs reports and statements filed with or furnished to the Securities and Exchange Commission and other information are posted on BDs website at www.bd.com/investors/.
Shareholders may receive, without charge, printed copies of these documents, including BDs 2007 Annual Report on Form 10-K, by contacting:
Investor Relations
BD
1 Becton Drive
Franklin Lakes, NJ 07417-1880
Phone: 1-800-284-6845
Internet: www.bd.com
Independent Auditors
Ernst & Young LLP
5 Times Square
New York, NY 10036-6530
Phone: 1-212-773-3000
Internet: www.ey.com
The trademarks indicated by italics are the property of Becton, Dickinson and Company, its subsidiaries or related companies. All other brands are trademarks of their respective owners.
Certain BD Biosciences products are intended for research use only, and not for use in diagnostic or therapeutic procedures. ©2007 BD
| Reconciliations to adjusted amounts (in millions) | 2007 | 2006 | ||||
| Operating income | $ | 1,203 | $ | 1,141 | ||
| Acquired in-process R&D | 122 | 53 | ||||
| Insurance settlement | | (17 | ) | |||
| Operating income adjusted | $ | 1,325 | $ | 1,178 | ||
| % change from 2006 | 13 | % | ||||
| as a % of revenues | 20.8 | % | 20.5 | % | ||
| Amounts may not add due to rounding. |
64